Ocuphire Pharma (OCUP.Q), a clinical-stage ophthalmic biopharmaceutical company focused on developing and commercializing therapies for treatment of several eye disorders, announced today that its VEGA-1 Phase 2 clinical trial has successfully met its primary and many secondary endpoints.
We are thrilled with the positive outcome in VEGA-1, which showed that a combination of Nyxol and low-dose pilocarpine produced a statistically significant improvement in near visual acuity in subjects with presbyopia…We would like to thank all of the subjects and investigational sites that participated in our first presbyopia clinical trial for Nyxol. Presbyopia represents an area of considerable unmet need due to its rising prevalence worldwide and the limitations of currently available corrective method,” said Mina Sooch, MBA, President and CEO of Ocuphire Pharma.
Presbyopia refers to the age-related loss of lens accommodation that results in an inability to focus at near distances. In other words, presbyopia is thought to cause universal near vision impairment with advancing age. According to the Community Eye Heath Journal, presbyopia is associated with substantial negative effects on health-related quality of life in the US population. Furthermore, presbyopia can cause a range of symptoms including eyestrain, headaches and perception impairment.
With this in mind, Ocuphire’s VEGA-1 Phase 2 trail is intended to evaluate the efficacy and safety of Nyxol, the Company’s preservative-free eye drop solution for presbyopia treatment. Announced today, the Company’s clinical trial evaluating Nyxol in combination with low-dose pilocarpine (LDP) in presbyopic subjects, generated positive results. More specifically, Nyxol has met its primary endpoint, having improved photopic binocular near vision at 1 hour in 61% of patients treated with Nyxol. Nyxol also achieved additional endpoints including rapid onset of efficacy at 30 minutes, sustained duration over 18 hours, and efficacy in both light and dark iris colors to name just a few.
“Based on the data generated thus far, we believe that Nyxol and LDP is novel in its mechanism of action and could become a leading pharmacological treatment option for presbyopia and potentially allow those afflicted to reduce their dependence on reading glasses. We plan to initiate our Phase 3 trials for presbyopia in 2022, building on our recent success of Nyxol for Reversal of Mydriasis with initiation of the second Phase 3 registration trial later this year,” continued Mina Sooch.
The vision care market was estimated at USD$66 million in 2019 and is expected to reach $93 million by 2027, registering at a CAGR of 4.5%. Currently, eye glasses make up the largest segment of this market due to their effectiveness, however, Ocuphire’s Nyxol eye drops could introduce a disruptive alternative to the eye care market. With positive Phase 2 results under their belt and a Phase 3 trial on the horizon, Ocuphire shows incredible potential as an upcoming pharmaceutical company.
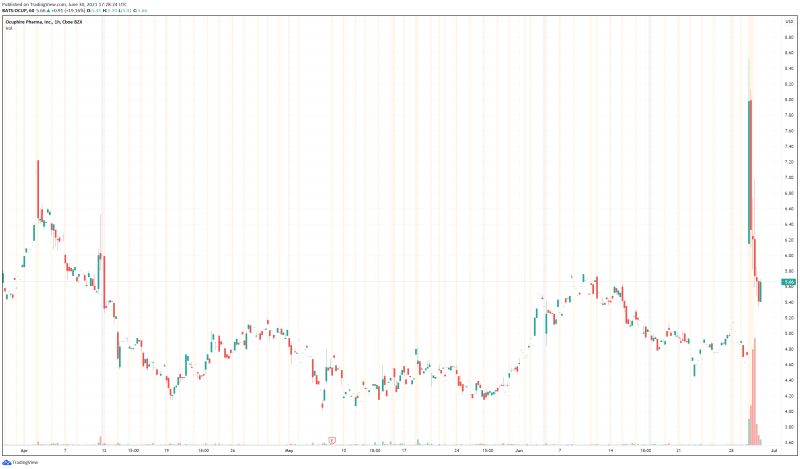
Ocuphire’s share price opened at $6.67, up from a previous close of $4.75. The Company’s shares are up 18.87% and are currently trading at $5.65 as of 1:30PM ET. This indicates that there has been significant change following the news.

