Lexaria Bioscience (LXX.C) has received test results for two new DehydraTECH 2.0 cannabidiol (CBD) formulations in its second 2021 applied research and development study program, HYPER-A21-2.
“One of our latest DehydraTECH 2.0 formulations gave us the strongest absorption enhancement results we’ve ever recorded, at 2,708 per cent more CBD into bloodstream during the study period than the representative industry standard MCT control formulation. It was also 174 per cent more effective than the original DehydraTECH 2.0 formulation from 2019…This is a 27-fold improvement in CBD delivery compared to the control formulation,” said Chris Bunka, chief executive officer of Lexaria.
Lexaria’s HYPER-A21-2 study included three new additional DehydraTECH 2.0 formulations. These formulations are intended to enhance CBD delivery performance and pharmacokinetic optimization. Put into layman’s terms, pharmacokinetic optimization refers to the optimization of drug activity in the body over a period of time. This includes the processes which drugs are absorbed, distributed, localized, and excreted. Scientific mumbo jumbo aside, two of the three new DehydraTECH 2.0 formulations delivered vastly improved performance when compared with both the Company’s original DehydraTECH 1.0 and 2019 DehydraTECH 2.0 concentration-matched formulas.
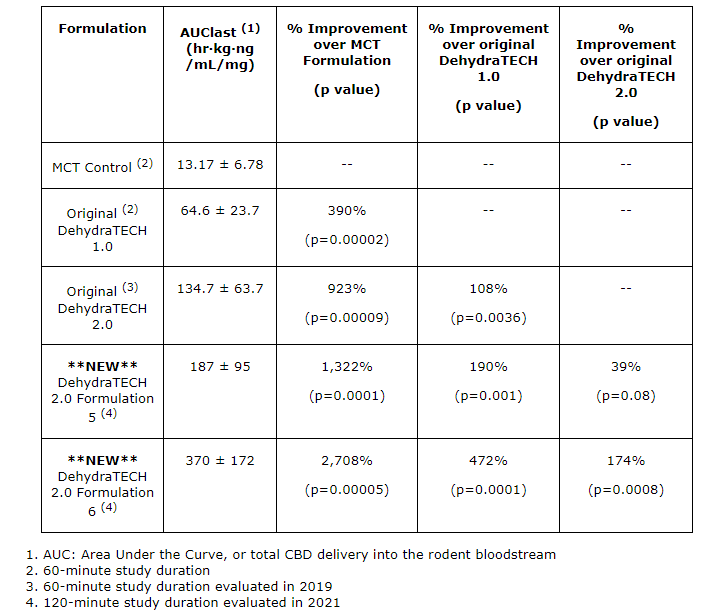
As referenced in the chart above, Lexaria’s latest DehydraTECH 2.0 formulations showed improvements in almost every category compared to its preceding formulations. I’m no scientist, but a 2,708% improvement in CBD absorption seems like a pretty staggering achievement to me. However, both of the Company’s HYPER-A21-1 and HYPER-A21-2 studies were conducted on 120 animals including rats.
With that being said, Lexaria has announced that its HYPER-H21-1 human clinical hypertension study is currently underway. Furthermore, dosing is nearly complete, utilizing a formulation closely resembling the original 2019 DehydraTECH 2.0. While results will likely differ from the Company’s animal trials, Lexaria believes its formulations will surpass the effectiveness of the original DehydraTECH 1.0 formulation used in the Company’s foundational 2018 human clinical study.
With this in mind, Lexaria plans to provide details on the outcomes of its HYPER-H21-1 human clinical studies when they become available. Investors and shareholders can expect to see some positive results in the future related to the performance of DehydraTECH 2.0 in human subjects. With its drug delivery technology, Lexaria could pave the way for innovative applications of DehydraTECH 2.0 in a wide range of areas including consumer packaged goods as well as drugs with potential for disease treatment applications. Furthermore, as referenced in a previous article, Lexaria’s DehydraTECH formulation has already demonstrated its unparalleled usefulness in maintain the stability of CBD beverages.
Lexaria Bioscience’s share price opened at $7.00, up from a previous close of $6.50. This Company’s shares are up 3.08% and are currently trading at $6.70 as of 12:27PM ET. This indicates that there has been some noticeable change following the news.