Revive Therapeutics (RVV.C) provided an update on their FDA Phase III clinical trial to evaluate the safety and efficacy of Bucillamine in patients with mild to moderate Covid-19.
Revive has partnered with 14 clinical sites and is now expanding to 50 sites, mostly in states that are currently Covid-19 hotspots. RVV says they are on track to meet their planned goal for the study in Q2 of 2021.
The study is a randomized, double-blind, placebo-controlled trial, and aims to enroll 1,000 patients. Their data is released to the Independent Data and Safety Monitoring Board (DSMB) every 200 patients, who use the results to evaluate the efficacy and safety of the drug.
So far, there have been no concerns that caused the DSMB to take action.
“We are making good progress both on enrollment and expansion of clinical sites in hot spot areas in the U.S. and we are positioned well to explore strategic initiatives in completing the Phase 3 study as well as seeking a path forward for EUA approval from the FDA,” stated Michael Frank, CEO of Revive.
Bucillamine was originally designed to treat arthritis, but when the Covid-19 pandemic hit, Revive found a new potential purpose for their drug. After a $23 million financing, they went to work on the Bucillamine Covid-19 trials.
They must believe things are going well, because they recently approached the FDA about an agreement to file an Emergency Use Authorization (EUA) application.
Getting an EUA would be a huge step forward and would allow doctors to start distributing Bucillamine.
Many might be thinking, with the vaccine being rolled out, that the Covid-19 pandemic is almost behind us, and so an EUA would be too-little-too-late at this point.
But they’d be wrong for two reasons.
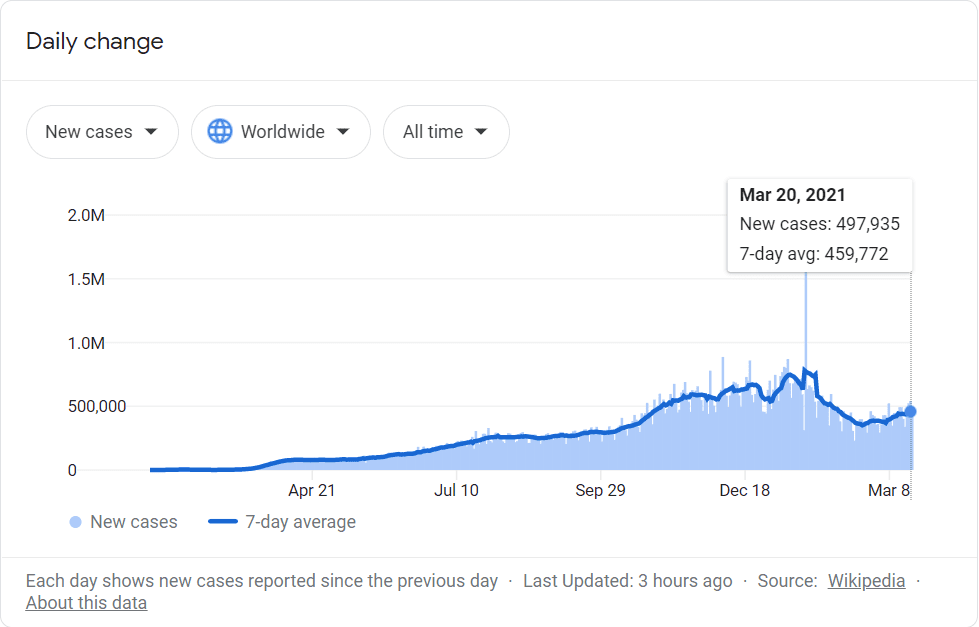
First of all, Covid-19 cases are nearly as high as they’ve ever been.
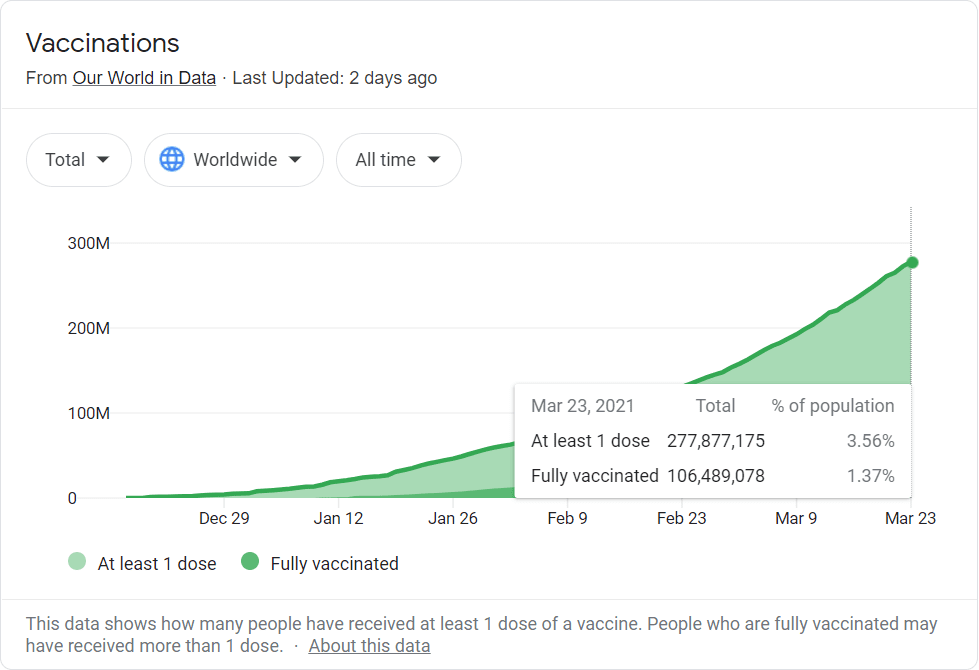
The US may be crushing it on the vaccine front, but less than 2% of the world’s population is fully vaccinated. And other countries may take longer to get hold of vaccines – something we know all too well up here in Canada at the moment.
The second reason they’d be wrong is that the Covid-19 vaccines are great at stopping people from getting severe Covid cases, but they are not 100% effective at stopping people from getting the illness at all.
Therefore, even in a world where 70% of the population is vaccinated, there may still be some use for a treatment that helps with mild to moderate coronavirus cases.
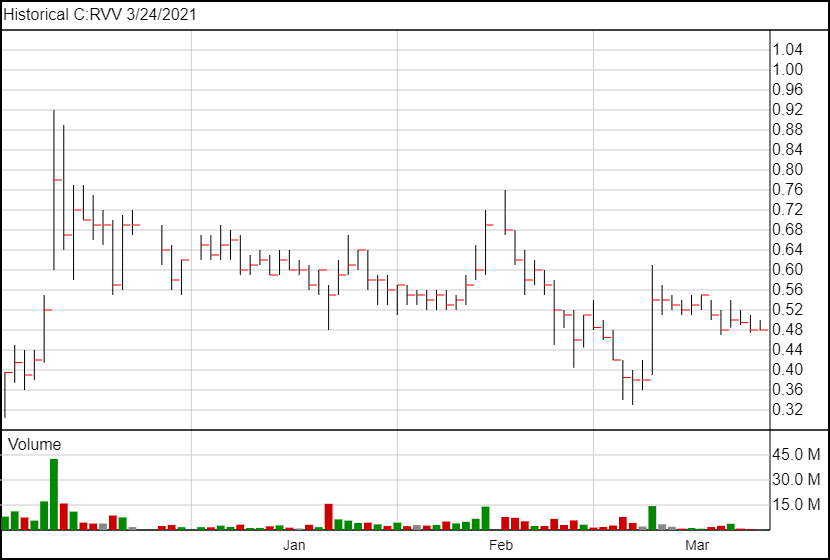
The stock price has not moved significantly following the news.
Full disclosure: Revive Therapeutics is an Equity.Guru marketing client.