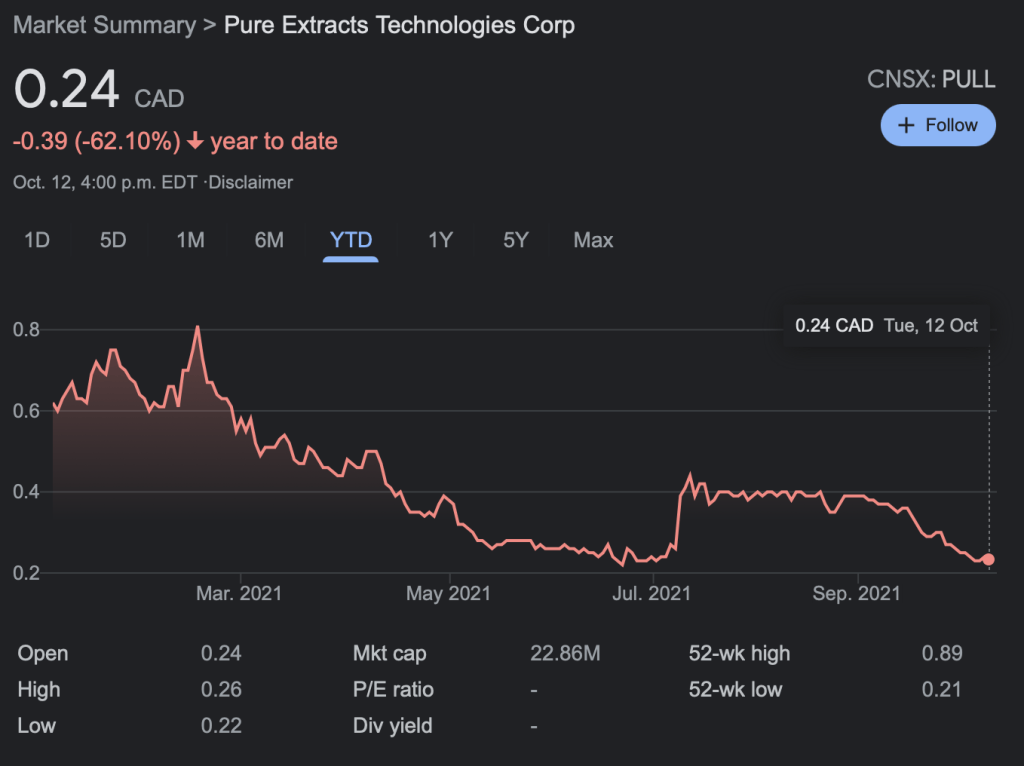
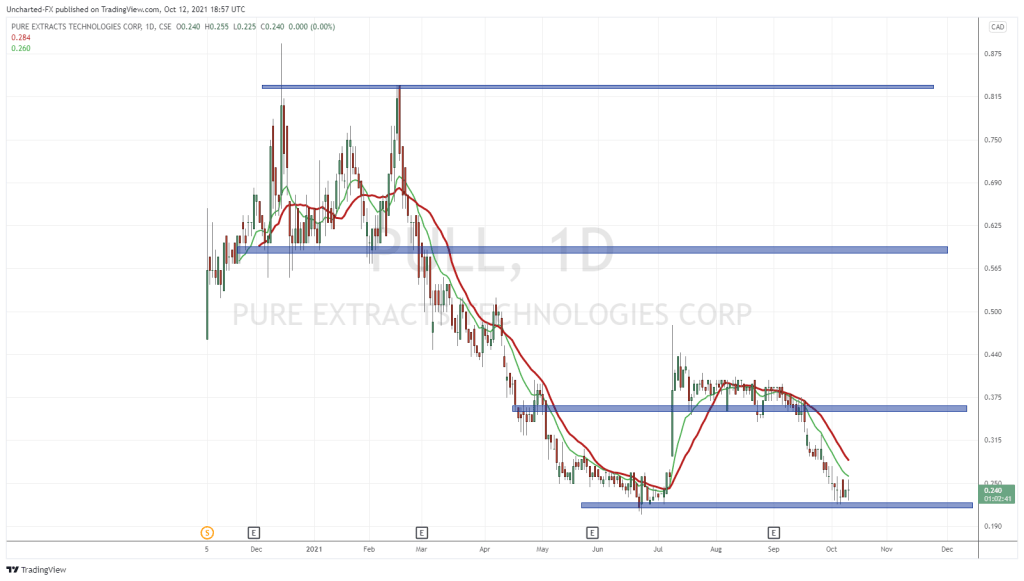
Pure Extracts Technologies (PULL.C) commenced a study on the formulation and manufacturing of psilocybin-based treatments today, according to a press release.
The products will come as oral tablets, capsules and a nasal gel for potential use as an investigational product. The study’s focus will be on the formulation, manufacturing and maximizing bioavailability potential for rapid onset dosage of psilocybin that could be used in future efficacy trials by both PULL and its customers.
“Having the support of TIPT, one of Canada’s premiere pharmaceutical R&D companies, while waiting to receive our Dealer’s License from Health Canada is invaluable. We are very excited to be laying the groundwork for our move into the controlled substances world of psychedelics and to be furthering our knowledge-base in psilocybin and associated novel delivery mechanisms,” said Ben Nikolaevsky, CEO for Pure Extracts.
Packaging, labelling and clinical batch manufacturing will be included in the study, as well as conformity and both long term and accelerated stability testing. The study will also conform to Canadian Good Manufacturing Practice and Good Clinical Practice standards, and by conducted at the Toronto Institute of Pharmaceutical Technology by Doctor Alexander MacGregor, a scientific advisor for the company.
Doctor MacGregor is a recognized expert in the field of pharmaceutical technology and novel drug delivery systems. He’s received several global patents for medical treatments and pharmaceutical drug delivery technologies, with notable countries including Canada, the United States, Australia, Europe, Latin America, China, India and Japan.
—Joseph Morton