On September 8, 2020 XPhyto Therapeutics (XPHY.C) announced that it is collaborating with its exclusive diagnostic partner, 3a-Diagnostics GmbH, to develop a “rapid, disposable, point-of-care lateral flow screening test to detect COVID-19 viral RNA from patient saliva samples and nasal and throat swabs.”
This follows a July 6, 2020 announcement that its 3a-Diagnostics is developing an affordable, portable screening tool that only requires the collection of saliva.
That means you wouldn’t need a Registered Nurse to penetrate your skull with a Q-tip – to a get a sample.
“We believe that a low-cost, portable and easy to use screening tool that provides rapid on-the-spot results would be a disruptive tool in the fight against pandemic threats,” stated Hugh Rogers, CEO of Xphyto on July 6, 2020. “We see an enormous global market opportunity that includes individual households, schools, hospitals, public transportation, airports and border services as well as many private employers.”
Patients infected with an alternate coronavirus strain or a highly mutated form of COVID-19 are expected to activate only the universal coronavirus probes. These patients could be selected for further investigation.
On Aug. 10, 2020, the company announced commercial milestones targeting European regulatory approval in Q1 2021.
- 3a has isolated COVID-19 RNA from live viable virus for its 2nd round of proof-of-concept prototype testing.
- Evaluation process is underway.
- Results are expected within 30 days.
- In Q4, 2020 – pending successful evaluation results – Xphyto will begin advanced prototype production.
- Expedited development and optimization at 3a’s research lab
- Ongoing collaboration with 3rd party contractors and academic partners in Germany.
“The scientific understanding of the COVID-19 virus and an active infection is rapidly evolving,” stated Rogers, “It’s a dynamic situation but Xphyto is bolstered by the emerging scientific literature that supports the use of saliva tests over nasopharyngeal swabs and molecular (RNA) tests over other forms of detection, which may be susceptible to false negatives.”
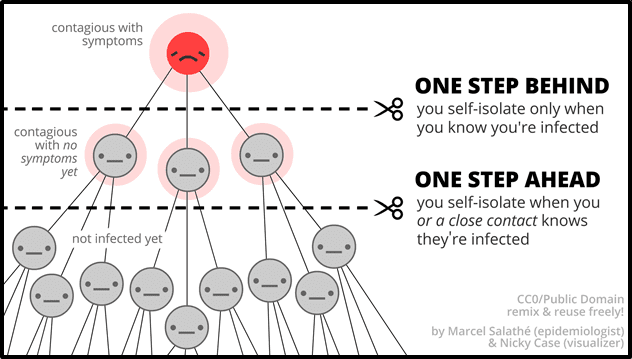
Xphyto and 3a are developing rapid screening tests for COVID-19 and other high-risk pandemic threats, including H1N1 (swine flu) and H5N1 (avian flu), with a specific focus on early pre-symptomatic and asymptomatic stages of infection.
Identifying “pre-symptomatic and asymptomatic stages of infection” is a big deal.
“Silent disease transmission during the pre-symptomatic and asymptomatic stages are responsible for more than 50% of the overall attack rate in COVID-19 outbreaks,” states The National Academy of Sciences.
“Our results indicate that the majority of transmission is attributable to people who are not exhibiting symptoms, either because they are still in the pre-symptomatic stage or the infection is asymptomatic”, the academy continued, “immediate isolation of all symptomatic cases is insufficient to achieve control”.
In Canada, about 1 in 260 people have contracted the coronavirus. Our actions are governed by crude unconscious statistical analysis.
Your life-time chance of dying in a car crash is 1%. Think about that for a minute, it might stop some people driving.
But when you amortize the risk over a 77-year-life, the odds of dying this year, fall to 1 in 7,700 (.013%) – not really worth fussing about – so we all keep climbing into cars, hoping for the best.
Asymptomatic coronavirus carriers often don’t take adequate precautions (masks, social distancing, bubbles) because the 1/261 chance of contracting the virus feels too small to worry about.
A cheap saliva test could be a game-changer in the global fight against this pandemic – and future pandemics.
H1N1 and H5N1 development programs are currently funded through grants from the German Federal Ministry of Education and Research.
Xphyto’s screening tests include enhanced RNA-probe lateral flow assay tests as well as novel biosensors delivered via Xphyto’s oral dissolvable drug delivery platform.
The product pipeline consists exclusively of next-generation rapid, low-cost, easy-to-use, saliva-based screening tools designed to be self-https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered, making them ideal for decentralized population-scale screening.
“Successful incorporation of 3a’s peptide biosensors into Vektor’s ODF platform represents a significant technical milestone on the pathway to commercialization,” stated Rogers. “This was a critical step that unlocks a pipeline of potential biosensor screening products.”
3a has developed peptide-based biosensor screening tests for bacterial and viral infectious diseases, including
- Influenza A
- Scarlet fever
- Stomatitis
- Periimplantitis
- Periodontitis
With formulation development and Oral Dissolvable Thin Film Oral Dissolvable Thin Film (ODF) Platform incorporation complete, Vektor and 3a are reviewing ODF optimization priorities such as: placement (tongue vs. cheek), size, dissolvability timeline and taste.
Vektor has begun strategic planning for pilot-scale EU GMP (European Union good manufacturing practices) test manufacturing for use in clinical evaluation.
“We believe that low cost, distributable and decentralized screening tests will be a critical component of global population-scale pandemic management,” stated Dr. Heinrich Jehle, Managing Director of 3a.
– Lukas Kane
Full Disclosure: Xphyto is an Equity Guru marketing client.







