Sernova Corp.

- $435.182M Market Capitaliztion
Sernova Corp. (SVA.V) has provided a progress update on the Company’s Phase 1/2 type 1 diabetes (T1D) clinical trial in conjunction with Dr. Piotr Witokski, the clinical trial’s Principal Investigator at the University of Chicago. Dr. Witowski was originally scheduled to provide a clinical trial update at the 2022 American Society of Transplant Surgeons (ASTS) Winter Symposium. However, the conference has since been postponed until August 2022 as a result of the ongoing COVID-19 pandemic.
“We are very encouraged by finding detectable serum C-peptide levels after islet transplantation into the Sernova Cell Pouch in the three most advanced study patients. Additionally, we are very excited about the first two patients becoming insulin-independent and free from severe hypoglycemic events after a single supplemental intraportal islet infusion…I look forward to continuing this trial with Sernova as we further optimize our use of the Cell Pouch System and validate its therapeutic potential,” said Dr. Witkowski.
Before we delve into Sernova’s latest news, let’s talk a little bit about the Company first. Sernova is a clinical-stage regenerative medicine technology company committed to the development and clinical advancement of its products for metabolic, hematological, and other chronic diseases. Sernova believes in advancing its clinical programs as well as building collaborations and partnerships intended to accelerate the Company’s portfolio of products into the market. To date, Sernova has partnered with BIOTECanada, Alliance for Regenerative Medicine, and the Biotech Innovation Organization, to name just a few.
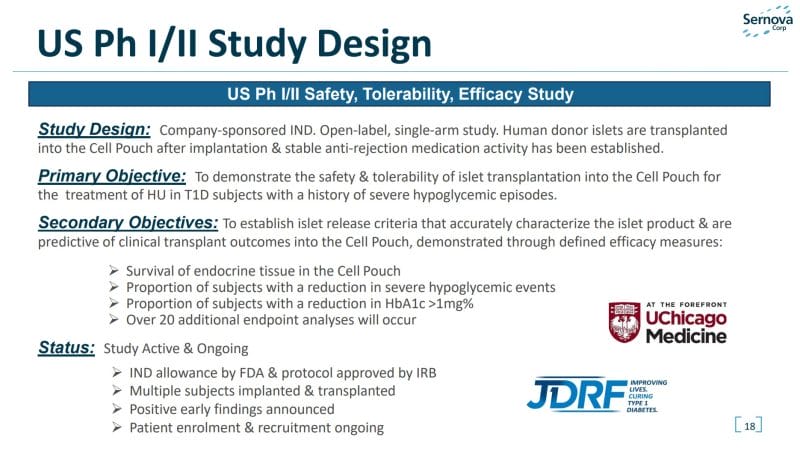
Cell Pouch System™
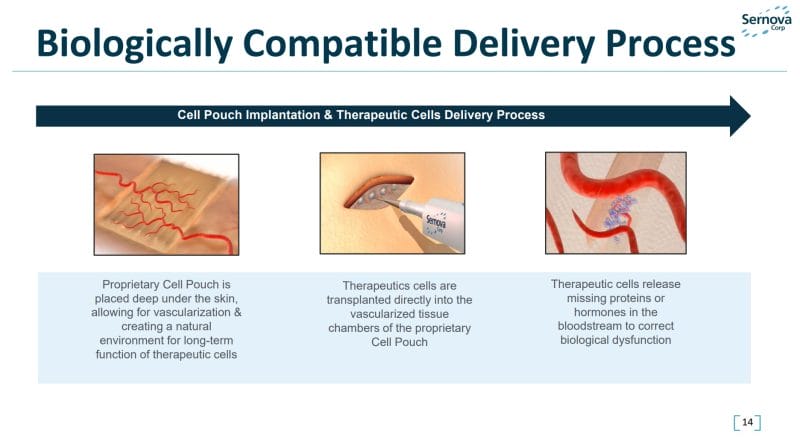
Sernova’s Cell Pouch System™ is a novel implantable and scalable medical device that forms a natural environment in the body for the hosting, long-term survival, and function of therapeutic cells. For context, therapeutic cells are injected, grafted, or implanted into a patient in order to effectuate a medical effect. With this in mind, therapeutics cells implanted via Sernova’s Cell Pouch System release the necessary proteins or factors missing from the body to treat chronic diseases as an alternative to the daily administration of drugs. The Company’s Cell Pouch System is currently undergoing clinical trials in patients with diabetes.
However, Sernova’s technology may also provide an effective environment for the treatment of other chronic diseases such as hemophilia, human growth hormone deficiency, parathyroid hormone deficiency, and Parkinson’s disease. Additionally, the Company’s Cell Pouch System’s safety and biocompatibility have been demonstrated in multiple preclinical studies. This technology has also been tested successfully in humans through a small clinical study. Bearing all of this in mind, Sernova’s Cell Pouch System has received various certifications including ISO 13485, MDR2017/745, US FDA Quality System Regulations 21 CFR 820, and Canadian Medical Device Regulation.
Efficacy
Sernova’s Cell Pouch System, when transplanted with insulin-producing islets, demonstrated efficacy in three different small and large animal transplantation models of diabetes, where animals achieved sustained glucose control. In other words, animals involved in these various transplantation models demonstrated insulin independence. Before we move on, let me explain what an islet is since it’s relevant. The pancreas contains clusters of cells that produce hormones; these are called islets. Put simply, these islets are responsible for producing a range of hormones such as glucagon, insulin, and somatostatin.
With this in mind, transplanted islets within Sernova’s Cell Pouch System are able to produce insulin naturally through a feedback system linking glucose levels to the release of insulin. As such, this may result in the reduction or elimination of the need for insulin injections, ultimately resulting in insulin independence.
Sernova’s Phase 1/2 Clinical Trial
The objective of Sernova’s Phase 1/2 clinical trial is to assess the safety, tolerability, and efficacy of its Cell Pouch System transplanted with insulin-producing islets in patients with type 1 diabetes (T1D) complicated by hypoglycemia unawareness and a history of severe hypoglycemic events. Hypoglycemia is a condition in which one’s glucose level is lower than normal. Some severe signs and symptoms of hypoglycemia include muscle weakness, drowsiness, unconsciousness, and confusion, among many others.
With this in mind, hypoglycemia unawareness (HU) refers to a person’s inability to recognize the symptoms of low blood sugar before they become severe or even fatal. HU typically occurs when blood glucose levels are below 3.0 mmol/L and is estimated to affect approximately 15% of people with T1D. Keep in mind, after a person has experienced their first HU episode, more are likely to occur. Referring back to Sernova’s Phase 1/2 clinical trial, study patients must meet stringent eligibility criteria.
Criteria include, but are not limited to, long-standing T1D, recent episodes of HU, and an absence of glucose-stimulated C-peptide, a substance made in the pancreas, along with insulin, detectable in their bloodstream. Study patients are eligible to receive up to three islet cell transplants throughout the study, including two marginal dose transplants into the Cell Pouch and a single, marginal islet supplement transplanted via the portal vein, a vein conveying blood to the liver from the spleen, stomach, pancreas, and intestines.
In Sernova’s latest press release, the Company highlighted study findings and key points from its ongoing trial:
- the ongoing safety and tolerability of Cell Pouch has been maintained in all study patients
- Islet transplantation to the Cell Pouch resulted in the establishment of new, measurable islet function documented by detectable levels of stimulated C-peptide in the first three patients, who completed the protocol-defined course of transplants
- a supplemental, single intraportal islet transplant was sufficient for the first two patients to achieve and maintain sustained ongoing insulin independence and freedom from severe hypoglycemic events for over 21 and 2 months, respectively
- the third transplanted patient recently completed their course of Cell Pouch transplants and a supplemental intraportal islet infusion, with favorable improvements in glucose control, near-normal levels of C-peptide, an absence of severe hypoglycemic events, and reductions in daily insulin use
- the other three enrolled study patients are progressing through the study protocol, as planned. All have received Cell Pouch implants and are at various stages of protocol-defined islet transplants and follow-up
- a 7th study patient has been identified
“We believe Sernova is the first company to report that its first two transplanted T1D cell therapy study patients achieved sustained insulin independence. We are further reassured that our Cell Pouch System is performing as expected by creating a safe, vascularized, and natural tissue environment inside the human body, allowing the islet cells to thrive and function efficiently following transplantation…
These findings have led to several technical optimizations, including the upcoming introduction of a higher capacity Cell Pouch configuration designed to accommodate the total quantity and distribution of islets that are achieving insulin independence for patients in our clinical trial,” commented Dr. Philip Toleikis, President & CEO of Sernova Corp.
Financial Results

According to Sernova’s Financial Statement for the three and nine months ended July 31, 2021, and 2020, the Company had cash of CAD$29,888,088 on July 31, 2021. This represents a significant increase from CAD$3,949,412 on October 31, 2020. Sernova’s total assets and total liabilities both increased to CAD$31,321,105 and CAD$1,087,120, respectively. For the three months ended July 31, 2021, the Company reported a net loss of CAD$1,630,998. For the nine months ended July 31, 2021, Sernova’s reported net loss was CAD$4,790,196.
Upcoming, on August 16, 2022, 7,242,375 outstanding warrants at an exercise price of $0.30 will expire. On February 3, 2021, Sernova announced that it had entered into an agreement with Canaccord Genuity Corp. and Leede Jones Gable Inc. (“Underwriters”), pursuant to which the Underwriters agreed to purchase, on a bought-deal basis, 8,350,000 units of Sernova at a price of CAD$1.20 per unit for gross proceeds of approximately CAD$10,020,000.
Shortly after, on February 4, 2021, pursuant to an amendment, the Underwriters agreed to increase the size of the initial bought-deal financing. According to the amendment, the Underwriters agreed to purchase an aggregate of 16,700,000units of Sernova at a price of CAD$1.20 per unit for gross proceeds of CAD$20,040,000. Sernova announced the closing of its bought-deal financing on March 1, 2021, for aggregate gross proceeds to the Company of approximately CAD$23,046,000.
As a whole, the global market for Diabetes Care Products, including drugs and devices, is expected to exceed $111.2 billion by 2027, expanding at a compound annual growth rate (CAGR) of 3.9%. This market is primarily driven by a global rise in the prevalence of obesity and unhealthy lifestyles. Currently, Type 1 and Type 2 diabetes are the two main types of diabetes, with Type 2 diabetes accounting for more than 85% of total diabetes prevalence. With this in mind, there is a sizeable market for Sernova’s Cell Pouch System, which has now demonstrated insulin independence through the Company’s Phase 1/2 clinical trial.
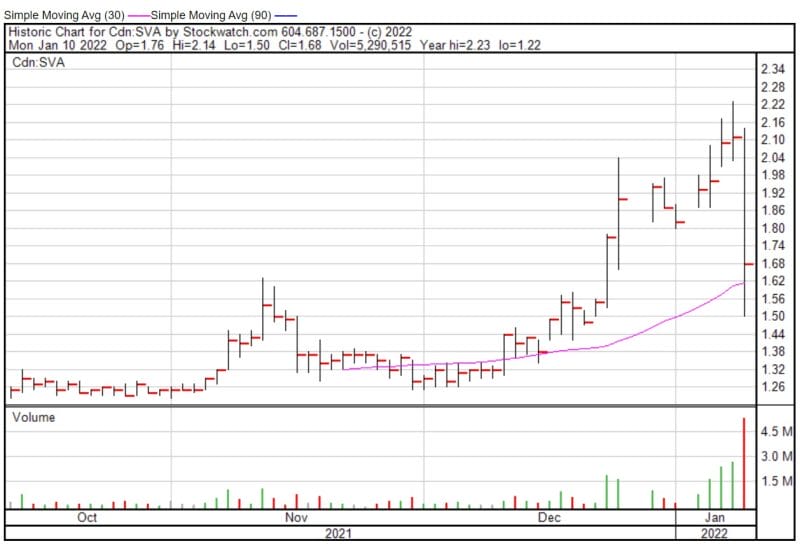
Sernova’s share price opened at $2.14 today, up from a previous close of $2.12. The Company’s shares are down -21.23% and were trading at $1.67 as of 12:46 PM EST.