Bioasis Technologies (BTI.V) announces today that the Japanese Patent Office (JPO) has issued Japanese Patent No.2019-189551 Notice of Allowance for the Company’s xB3™-IDS fusion proteins.
“We are excited about the acceptance of this patent by the Japanese Patent Office. It represents a major milestone for our intellectual property protection around our core assets for the delivery in the Lysosomal Storage Disease area in the important Asian market, particularly the Japanese market,” said Dr. Deborah Rathjen, Executive Chair of the Board of Bioasis.
Bioasis latest patent relates to fusion proteins between the Company’s xB3™ and Induronate-2-sulfatase (IDS), as well as compositions and methods related to the use thereof. If you’re new here, that probably means nothing to you, so let me explain. Bioasis is a biopharmaceutical company credited for the development of its xB3™ platform, a proprietary technology for the delivery of therapeutics across the blood brain barrier (BBB) in order to treat central nervous system (CNS) disorders, including brain cancers and neurodegenerative diseases. For context, the BBB is responsible for protecting against toxins or pathogens that could cause brain infections, while simultaneously allowing vital nutrients to reach the brain. In other words, the BBB is like the world’s most qualified club bouncer, preventing the entry of zoomers who have yet to graduate from high school…no offence to our teen readers.
The issued claims of Bioasis’ most recent patent cover the compositions for xB3™-IDS fusion proteins and the treatment of lysosomal storage diseases (LSDs) at risk of developing CNS involvement, including Hunter Syndrome (MPS II). Let me give you a crash course on LSDs (not the drug). Lysosomal storage diseases are inherited metabolic diseases characterized by an abnormal buildup of various toxic materials in the body’s cells s a result of enzyme deficiencies. As previously mentioned, this disorder can develop into Hunter Syndrome, an inherited disorder in which the body does not properly digest sugar molecules, resulting in damage that affects physical and mental development.
In addition to the already granted patents worldwide, this further strengthens and reinforces our standing around our xB3™ platform technology, particularly in the Lysosomal Storage Disease area,” continued Dr. Deborah Rathjen.
As a group, LSDs are believed to have a frequency of about one in every 5,000 live births. In particular, Hunter Syndrome, which is almost always diagnosed in males, has a prevalence of roughly 1 out of 100,000 to 170,000. With this in mind, the global LSD therapeutic market is expected to read a valuation of USD$14.36 billion by 2026, expanding at a CAGR of 10%. Currently, methods of LSD treatment include enzyme replacement therapy (ERT), bone marrow transplantation, substrate reduction therapy, use of molecular chaperones, and gene therapy. The most effective therapeutic strategy for LSDs is to replace the missing enzyme, however, because of the BBB, no effective ERT is currently approved to treat CNS manifestations like Hunter Syndrome. That’s where our good buddy Bioasis comes in with its xB3™ platform, which is intended to deliver ERT across the BBB and into the brain.
Bioasis patent follows the June 18, 2020, Australian patent application No. 2015219339 granted by the Australian patent office, also relating to the IDS polypeptide. For a more detailed article covering Bioasis’ financials and pipeline, feel free to check out this article.
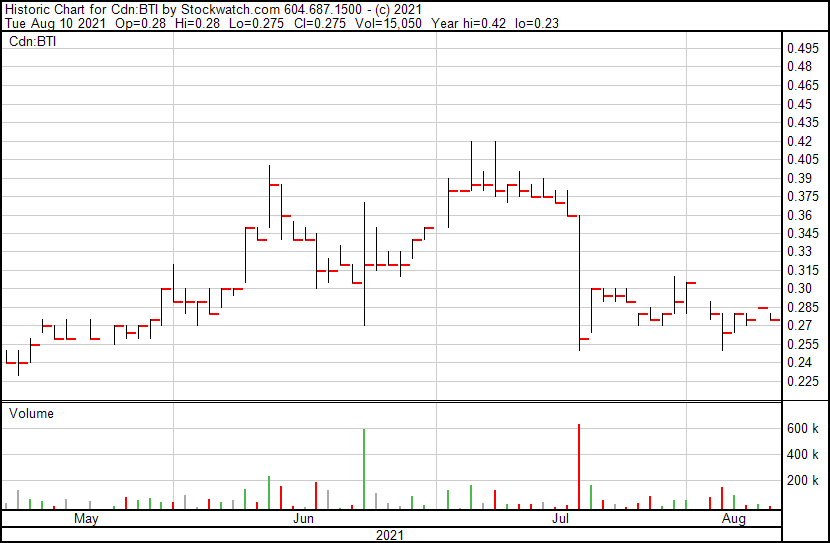
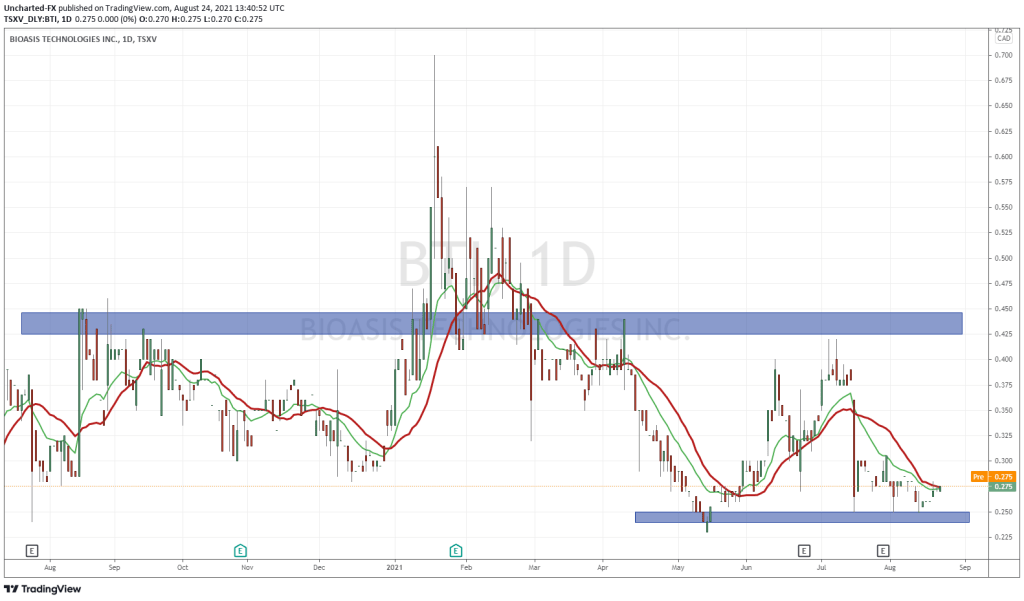
Bioasis’ share price opened at $0.28, down from a previous close of $0.285. The Company’s shares are down -1.75% and were trading at $0.28 as of 10:27AM ET.
Full Disclosure: Bioasis is a marketing client of Equity Guru.