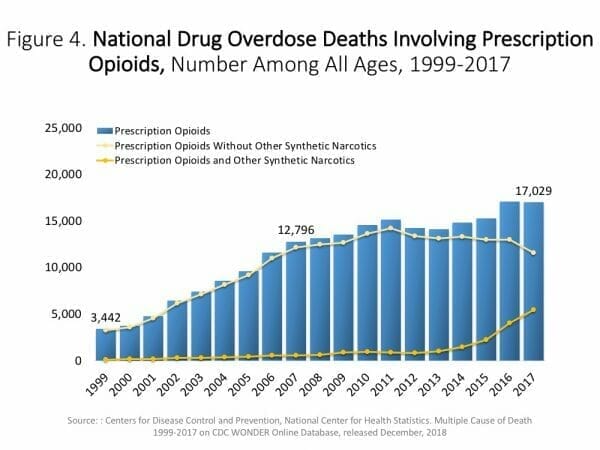
Oxycontin, once pitched as a treatment for chronic pain, is now synonymous with the opioids currently plaguing Canada and the United States.
In the U.S. alone, some estimates place the number of deaths resulting from prescription opioids like Oxycontin as high as 200,000 since 1999.
The death toll rises when factoring in the number of overdoses from street drugs, the likely alternative for users who could no longer obtain or afford their prescriptions.
Purdue Pharma, Oxycontin’s manufacturer, is currently embroiled in a lawsuit launched by Massachusetts’ attorney general over the overdose epidemic it helped create.
Whether the company is ever punished for their careless disregard for human life is largely irrelevant given the terrible cost to date, though there is something to be said for the scathing indictment of our negligent physicians, the stewards of our health who simply looked the other way.
And while the opprobrium of executives is well-earned, court rulings are a poor salve for those still in the grips of addiction, and poorer still for the dead.
What the opioid crisis–and the resulting backlash–has done, besides ravaging large swathes of already economically depressed regions, is demonstrate the overwhelming public desire for a shift in the Overton window of acceptable drug treatment.
We now know social stigma inhibits one’s willingness to seek treatment for drug addiction. And the discourse surrounding mental health issues, only now being broached by mainstream media outlets, is still in its infancy.
Although it is clear there is work to be done in both cases, the question remains: what about an evolution in the drugs themselves?
It’s clear the old ways aren’t working, and it’s reflected in the way Big Pharma is percieved. A 2016 poll found only 9% of U.S. consumers believe pharmaceutical and biotech companies prioritize patients over their bottom line.
Drugs have one purpose: to improve one’s quality of life. When they are ineffective (like antidepressants), or lethal in the case of Oxycontin, there is an inefficiency. Something has to change.
Rethinking Opioid Use Disorder
Some pharmaceutical companies are conscious of the desire to rethink treatment methods, and Titan Pharmaceuticals (TTNP.Q) is one of them.
The company has developed a vehicle for a six-month treatment for Opioid Use Disorder (OUD) known as Probuphine, a compound which has fewer harmful side-effects of drugs like Methadone.
Probuphine is a subdermal–below the skin–implant which continuously releases buprenorphine over the course of six months. Buprenorphine is the gold standard for opioid treatment drugs, controlling withdrawal symptoms without inducing the ‘high’ of drugs like methadone. In fact, buprenorphine prevents it.
As mixed partial agonist-antagonist, buprenorphine selectively binds to the opioid receptor and blocks opioid molecules from attaching to it.
“If you use heroin, for example, or if you use heroin or Vicodin or Percocet, you are not going to get high because the buprenorphine molecule is blocking that opioid receptor from being turned on,” said Kate DeVarney, Titan’s executive vice president and CSO.
With rare exception, methadone can only be https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered under supervision, whereas buprenorphine can be prescribed and taken unsupervised.
There’s also a psychological component: taking medication every day may trigger previous substance abuse behaviours, prompting the seeking out of illicit drugs.
DeVarney said Titan’s implant addresses a number of issues within opioid treatment. Because it’s an implant rather than a pill, the implant releases a predetermined amount every day. Probuphine can’t be resold or abused through intentional over-dosing.
This isn’t your mother’s medication
But innovations within the pharmaceutical field right now aren’t just limited to form. The entire Overton window for recognized drug treatments is shifting.
For instance, Time published an article in 2017 about the new forms of treating depression. One of these methods involved the use of Ketamine, a widely-used street drug popular on the rave scene.
“Since ketamine is FDA-approved as an anesthetic, physicians can legally prescribe it off-label for any condition they believe it may help, including depression.”
A new study from the Chicago College of Medicine in Illinois showed two-thirds of participants who did not respond to traditional antidepressants experienced “fast and lasting resolution of their depressive symptoms after being given ketamine intravenously.”
Used primarily in veterinary surgery, Ketamine manufacturers like Mylan N.V. (MYL.Q) may have unknowingly developed a treatment for depression, a mental illness which one in six Americans currently take medication for.
According to Bloomberg, ketamine-based antidepressants may soon be making their way to store shelves in the U.S. The Food and Drug Administration is on the verge of approving an esketamine spray by Johnson & Johnson (JNJ.NYSE) after a panel overwhelmingly voted that the drug’s benefits outweighed the risks of abuse.
Breaking orthodoxy
Sensing the demand for alternative forms of treatment, Compass Pathways, a private company started by Peter Thiel, is researching the benefits of psilocybin therapy in treating depression. Psilocybin is the psychedelic compound within magic mushrooms which causes euphoria and visual hallucinations.
“We are beginning a randomised controlled trial of psilocybin therapy for treatment-resistant depression. This will be the largest clinical study of its kind and will take place in a number of clinical trial sites across Europe.”
–Compass Pathways
The company says it aims to bring a pharma-grade product using psilocybin to market within the next five to 10 years.
Similarly, the Multidisciplinary Association for Psychedelic Studies (MAPS), is currently running trials on the efficacy of MDMA in treating post-traumatic stress disorder.
Rick Doblin, MAPS’ founder and executive director, outlines his organization’s progress towards legalizing MDMA-assisted psychotherapy in the video below.
The takeaway
The opioid crisis, and its aftermath, represents a cultural moment. What is now painfully obvious is that there is a need for innovation within the pharmaceutical industry.
Actors in the pharmaceutical space like MAPS and Titan are sensitive to this need for innovation, providing harm-reducing treatments for everything from anxiety to chronic pain while minimizing a patient’s exposure to detrimental effects.
Whether through our investment or in the checkout line, the drug companies we support must prioritize the patient’s health. Instead of innovative new ways to extract profits, we need a pharmaceutical industry which aims to find new ways to improve quality of life.
Otherwise, there will be another Oxycontin. And this epidemic won’t be the last.
–Ethan Reyes