Data protection
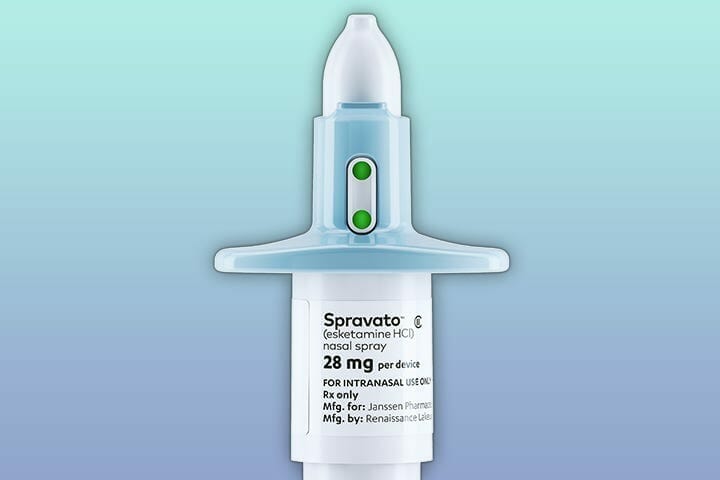
Earlier this week a federal review echoed The Canadian Ministry of Health’s original decision to deny data protection for Johnson & Johnson (JNJ.Q)’s Spravato product, a ketamine-derived NCE also known as esketamine. Spravato was deemed not unique enough for protection, which could raise some questions for other drug developers looking to get exclusivity on their future compounds.
In Canada, data protection provides eight years of exclusivity over data presented by the first company to commercialize a pharmaceutical product when applying for approval.
Data protection means that no generic manufacturer can market a drug product with the same active ingredient by relying on data submitted by the company that initially developed the drug.
Spravato
Spravato is a drug invented by J&J subsidiary Janssen that treats major depressive disorder in patients who have not responded to other antidepressant medications. The active ingredient in Spravato is esketamine hydrochloride, an enantiomer of ketamine hydrochloride. While ketamine hydrochloride was previously approved by Health Canada as an injectable anesthetic, circumstances surrounding the marketing authorization for esketamine hydrochloride differed substantially from the circumstances surrounding ketamine hydrochloride.
Patients with MDD who, despite trying at least two antidepressant treatments given at adequate doses for an adequate duration in the current episode, have not responded to treatment are considered to have treatment-resistant depression are then eligible for esketamine. The intention is that esketamine provides rapid relief from depression symptoms until the other medication takes effect.

Esketamine is only part of the molecular structure of pure ketamine, but Johnson & Johnson poured a shit ton of money into proving esketamine would be useful in treating depression. Unfortunately during the trials, esketamine was never put up against pure ketamine, JNJ didn’t want to create any potential variables that could mess up the results of their baby. The foundations of these trials began back in 2013, long before the new psychedelic wave took off.
Spravato was evaluated for efficacy and safety in more than 1,700 adult patients (18 to 86 years) who met DSM–5 criteria for MDD and were non–responders to at least two oral antidepressants. The development program included five Phase 3 studies (three short-term and two long-term studies) and one Phase 2 dose-ranging study. In the induction study, those who took Spravato and an oral antidepressant experienced a statistically significant improvement in depression symptoms at four weeks compared to those who received a placebo and an oral antidepressant.
So their bet paid off and the FDA approved Spravato for depression, although, with some pretty harsh restrictions. For example, a patient taking Sparvato has to have tried two SSRIs prior to getting Spravato treatments
See also: Ketamine vs. esketamine, why has the FDA only approved one for depression
Research into ketamine has long been paused due to its legal status, and because it’s not patentable, it’s also not as profitable as something more unique like esketamine. Ketamine clinics across North America use Spravato over pure ketamine to work within the legal framework the FDA’s decision created. I have written about the profitability of these clinics and how they have become a focal point of the psychedelics sector in 2021 for early revenue generation.
Novamind’s (NM.C) Utah-based clinics put up an impressive $1.86 million CAD in revenue last quarter, and the gross profit on that revenue was $1.37 million CAD.
See also: Novamind’s (NM.C) stock price makes zero sense right now
Unfortunately for Johnson & Johnson, the drug is not unique enough, at least not in Canada. However, since Spravato is also protected by patent rights, Janssen’s product could be protected from copycats through other legal means.
Future ramifications
What does this mean for Canadian drug developers looking to knock it out of the park with an NCE?
I’m not sure at this point, but it has to be some cause for concern. There is all this talk about who will make it to the finish line first with their clinical trials. Who will be able to be the first to market with a breakthrough drug that was created in-house or through IP acquisition? But maybe there should be more conversation around how will governing bodies like the FDA and Health Canada react when and if there are breakthroughs.
If a company’s value hinges on them having a unique drug that no one can copy they could be in trouble when given a similar fate as Johnson & Johnson’s Spravato. For them this is merely a drop in the bucket, they are a massive company that can afford mountains of litigation and has several other products to generate revenue and create company value.
One of the selling features of taking an early speculative bet on a psychedelics company is the possibility of them creating an exclusive NCE.
So for a smaller, say $20 million dollar market cap psychedelics company with all of its eggs in an NCE basket, this kind of news would be a pretty big blow to the company, its value, and likely its share price. It could also kill future M&A value as a big pharma would have a higher likelihood of being able to just create a version of it themselves.
This is another reason why it’s probably a good idea to have multiple drug candidates running through clinical trials. Companies are powerless as to how regulators are going to respond.
So where does Johnson & Johnson go from here?
The FCA determined that the Minister of Health’s decision-that SPRAVATO is a variant of a medical ingredient that had already been approved by Health Canada thus making it ineligible for data protection-was reasonable. Janssen has until September 29, 2021 to file an application seeking leave to appeal the FCA decision to the Supreme Court of Canada.