Oragenics Inc. (OGEN.NYSE) announced today that it has entered into a licensing agreement with the National Research Council of Canada (NRC).
“With respect to our potential future competitive positioning against currently available SARS-CoV-2 vaccines, we believe the licensed technologies will improve development speed, while the ability to rapidly engineer new vaccine antigens will permit us to quickly address new variants as they arise…,” said Frederick W. Telling, Ph.D., Oragenics’ Executive Chairman.
Oragenics is a development-stage company dedicated to fighting infectious diseases including coronaviruses and multi-drug resistant organisms. Currently, Oragenics is focused on the development of Terra CoV-2, the Company’s immunization product candidate intended to combat COVID-19 and SARS-CoV-2 variants. With this in mind, Oragenics’ latest agreement with the NRC will enable the Company to pursue the rapid development of next-generation vaccines including Terra CoV-2.
The NRC’s technologies in combination with elements found in Oragenics’ Terra CoV-2 vaccine, will provide the Company with a platform that can generate cell lines for high yield production of spike protein antigens for existing and emerging variants of concern. For context, a cell line is a cell culture, the process by which cells are grown, of a single type of cell that can reproduce indefinitely. While traditional production of cell lines can take between 6-9 months, Oragenics’ platform will be able to output cell lines within 6-8 weeks of spike gene sequence availability.
“…In addition, our agreement with Biodextris for an intranasal adjuvant is expected to complement our intramuscular https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration options and should position Oragenics with several antigen-adjuvant options in the event that SARS-CoV-2 become a seasonal flu-like disease, as many experts anticipate will be the case,” continued Dr. Telling.
According to Dr. Telling, this license will enable Oragenics to jumpstart IND-enabling animal studies with supplies of spike proteins to address the wild-type Wuhan virus as well as the Beta variant that is currently of global concern among public health officials. Furthermore, the Company recently entered into an agreement with Biodextris Inc., a contract research organization, on March 9, 2021.
Through this agreement, Biodextris will provide Oragenics with an intranasal adjuvant, a nasally https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered substance used to enhance the immune system’s response to the presence of an antigen. Biodextris’ nasal adjuvant will be used in combination with Oragenics’ antigen vaccine candidate as part of the preclinical immunological evaluation of the Company’s Terra CoV-2. Overall, Oragenics is well positioned to pursue the rapid development of next-generation vaccines. In Q1 2021, Oragenics was able to more than double its assets, achieving a total of $37,365,811 on March 31, 2021. Moreover, the Company was able to reduce its total liabilities to $1,315,344 in the same period. As of March 31, 2021, Oragenics’ cash and cash equivalents were sitting at $36,500,856.
Looking forward, the NRC’s support is expected to expedite the evaluation of SARS-CoV-2 antigen candidates in preclinical and clinical studies. With this in mind, preclinical studies were initiated in June in collaboration with the NRC, starting with an immunology study in mice to evaluate several adjuvant candidates in combination with Terra CoV-2. The best adjuvant candidate will advance into a hamster challenge study to assess inhibition of viral replication and and IND-enabling GLP toxicology study.
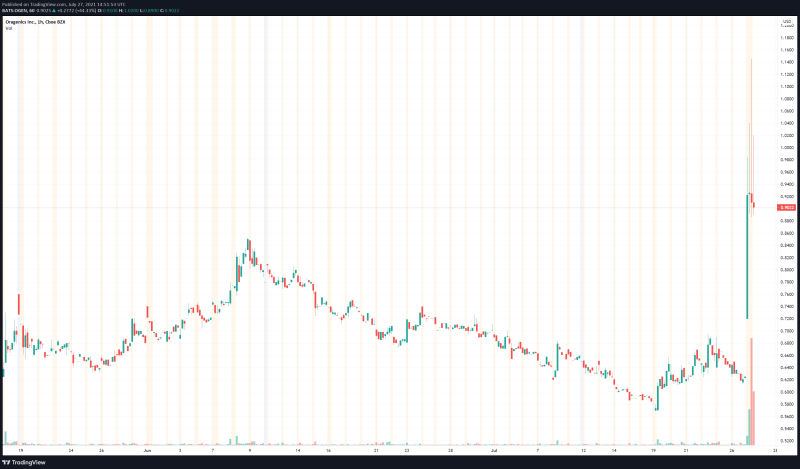
Oragenics’ share price opened at $0.62. The Company’s shares are up 54% and are currently trading at $0.96 as of 10:56AM ET. This indicates that there has been significant change following the news.

