Entheon Biomedical (ENBI.C) was born out of CEO Timothy Ko’s brother’s addiction-related death. Ko says the family spent a considerable amount of time and money over multiple years trying therapies that ultimately didn’t work. Ko, an entrepreneur recognized what was happening with psychedelics and mental health and decided to get into the sector.
Before Entheon Ko was the director of HyperBridge Technology – a blockchain management platform. And it looks Ko has brought his prior interest of tech into Entheon.
Through reading Entheon’s documents the company is extremely interested in all sorts of tech from VR to test kits to brain imaging technology. The company is also focused on new DMT delivery methods that would benefit both doctors and patients. They’re also working on a clinical trial testing DMT’s efficacy in treating addiction.
A new DMT delivery system
Entheon has roughly $4M CAD in working capital, a market cap of $17.8 million CAD with 54 million shares outstanding. With their working capital, Entheon is developing a new DMT delivery system and a number of DMT products.
The DMT Delivery System will include sensors to monitor the patients’ brain activity, along with heart rate, body temperature and other vital signs, to ensure that they are responding as expected to the treatment. The system will be https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered within a proprietary therapeutic protocol developed by Entheon. It’s intended to integrate intravenous infusion technology with real-time monitoring devices, including electroencephalography (EEG).
EEG is an electrophysiological monitoring method to record electrical activity on the scalp that has been shown to represent the macroscopic activity of the surface layer of the brain underneath. It is typically non-invasive, with the electrodes placed along the scalp.
EEG monitoring will help onsite clinicians assess a patient’s subjective experience, by transmitting sensitive measurements of neuronal signal strength and complexity that have been established as correlates of psychedelic immersion. From this research and data gathering initiative the objective will be to increase Entheon’s ability to develop therapies that are specific and responsive to an individual patient’s needs.
Unlike other psychedelic experiences, if the patient has an adverse reaction, Entheon’s DMT Delivery System will allow the experience to be stopped safely and quickly without the need for sedatives or other drug interventions. The system uses a pump-like action
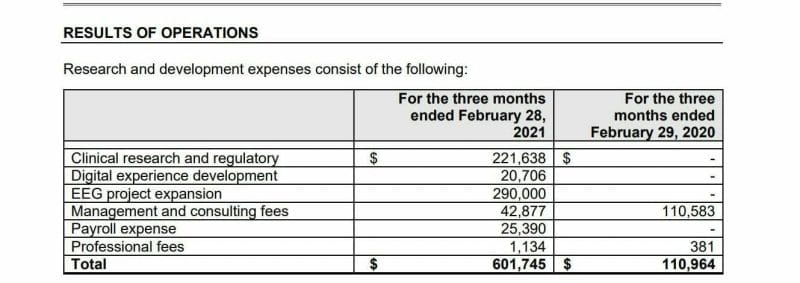
Entheon recorded R&D expenses for Q1 2021 of $601,745 CAD compared to $110,964 CAD in Q1 of last year primarily due to the development of products like the DMT Delivery System.
The company also has filed 4 patents for its DMT delivery systems.
Entheon does not currently generate revenue. Rather Entheon intends to generate revenue through the sale of its DMT Products, and eventually the license of its DMT Delivery System to physicians, clinics, and licensed psychiatrists in the United States, certain countries in the European Union, and throughout Canada.
Piles of DMT tech
Last month Entheon acquired Lobo Genetics for 5 million ENBI shares.
Lobo genetics operates a 5,000 sq/ft lab in Mississauga, Ontario, and has a team proficient in diagnostics, data, and analytics. They’ve got 8 employees listed on LinkedIn which also includes some engineers. Lobo has various existing distribution and partner relationships both domestically and internationally that are a strategic fit with Entheon’s goal of expanding its personalized psychedelics platform globally.
Entheon is also developing VR/AR-based digital products to aid in the psychedelic-assisted psychotherapy preparation, treatment, and integration process. The company believes these digital products will help acclimate people to the psychedelic experience.
In January, Entheon acquired HaluGen, a biotech company in the business of developing and commercializing a pre-screening test to identify genetic markers predictive of an individual’s reaction to hallucinogenic drugs.

The Psychedelics Genetic Test Kit gives its end users personalized reports and insights to their smartphone or computer through the HaluGen’s platform. The test also provides insights into the short and long-term potential of psychedelic-induced risks, such as psychosis. This is important for people like me who have a long family history of mental health and addiction. The first kit was released in June. Their MD&A from February says the company isn’t generating revenue, so I assume this is the company’s first product to hit the shelves and we will maybe see some revenue numbers later this year. I also wonder what kind of partnerships Entheon could land with this tech, like with the clinics themselves vs. trying to sell it B2C.
I’m sure that has already crossed their minds.
Entheon has a 5% controlling interest in Heading Health LLC. This investment into Heading Health provides Entheon with exposure to the ketamine-assisted therapy space, including Spravato, an FDA-approved Ketamine product that is eligible for insurance reimbursement.
See also: Ketamine vs. esketamine, why has the FDA only approved one for depression?
Clinical trials
Entheon isn’t just focused on DMT products, delivery systems, and patents – they are also partnering up to fund a clinical trial to try and prove DMT’s ability to aid in addiction. And Entheon isn’t the first ones down this path as John’s Hopkins already proved that psilocybin was useful in treating nicotine addiction. Cybin (CYBN.NE) is also studying psilocybin for alcoholism addiction.
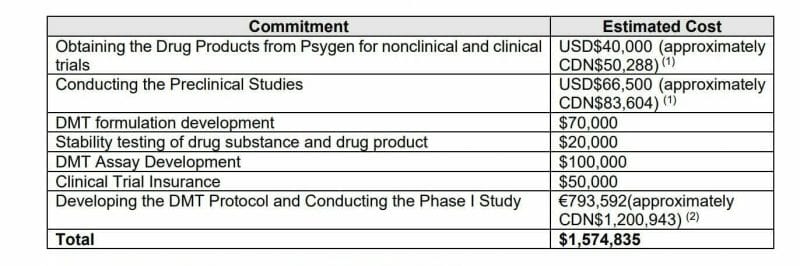
Entheon entered into a supply agreement with CHDR (the Centre for Human Drug Research located in Leiden, Netherlands) to perform a DMT-based phase I safety and proof-of-concept clinical study in humans. Entheon will pay $1.4 million CAD to CHDR for the phase I study. Upon completion of Phase 1 Entheon intends to complete an additional clinical study in the United States focused on addressing nicotine addiction.
The Phase 2 Nicotine Study is expected to compare measures of neuronal activity and complexity between subject groups, obtain cardiovascular and immunological health data, assess improvements in objective measures of wellbeing via interviews and questionnaires, evaluate frequency and severity of adverse effects, and determine the rate of nicotine use following DMT-assisted therapy.
The Phase 3 Study consists of an expanded pilot study to multiple clinics to assess treatment outcomes in larger populations of nicotine users, including specific indications.
This is a fast-paced company that has nailed down several partnerships in under a year, all of which appear to fit the company’s direction. They appear to always be looking for the next thing that could enhance what they’re building. They have an agressive M&A strategy but haven’t dilluted their company all that much, keeping a pretty tight share structure.

