Cognetivity Neurosciences (CGN.C) today announced the publication of the latest scientific paper on its Integrated Cognitive Assessment (ICA) in the peer-reviewed academic journal, Frontiers in Psychiatry.
“Empowered by AI, the ICA has the clear and exciting potential to achieve enhanced performance over time and to enable personalized medicine irrespective of geographic boundaries, as our new paper shows…The use of AI in decision making, especially for diagnostic decisions in healthcare, requires a level of explainability from the model which can be used to understand the important factors that lead to its output. This level of explainability is clearly achieved in the ICA. It can give clinicians full confidence in the platform, support improvements in system performance over time, and protect against the serious danger of bias,” said Dr Seyed Khaligh-Razavi, Cognetivity’s Chief Scientific Officer.
Don’t worry, I’ll spare you the trouble of having to read an entire academic journal, one of my favorite pastimes. Frontiers in Psychiatry’s latest publication provides evidence towards the validity of Cognetivity’s ICA test as a cognitive biomarker for detecting and monitoring patients with mild cognitive impairment (MCI) and mild Alzheimer’s Disease. Additionally, the paper reaffirms the ICA’s sensitivity in detecting cognitive impairment at the early stages of Alzheimer’s Disease, when treatment in known to be most effective.
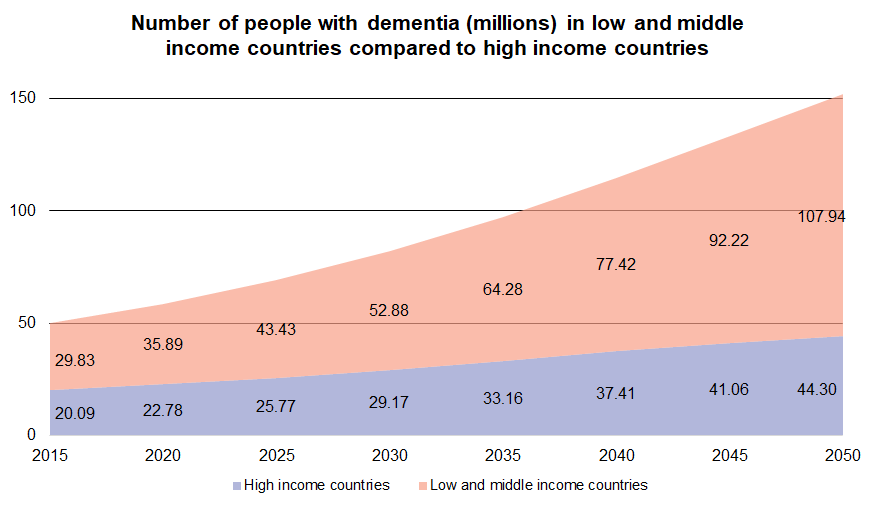
According to Alzheimer’s Disease International, there were over 50 million people worldwide living with dementia in 2020. Keep in mind, this number is expected to double every 20 years, reaching 82 million people in 2030 and 152 million in 2050. Statistically, this equates to someone in the world developing dementia every 3 seconds. To make matters worse, Alzheimer’s Disease is closely linked to genetics, putting those who have more than one first-degree relative with Alzheimer’s at greater risk. With this in mind, the need for a universal tool capable of detecting MCI and Alzheimer’s Disease at early stages has becoming increasingly prevalent.
Traditional cognitive tests, whether they are pen-and-paper based or computerized, require the collection of language specific data in new global locations before they can be deployed at scale. With this in mind, the latest publication in Frontiers in Psychiatry demonstrates the effectiveness of Cognetivity’s ICA in different international populations without the need to collect population-specific normative data in new environments.
Put simply, the Company’s ICA test is able to generate results that can be generalized across any population without any bias towards language, culture and education. Additionally, new data on Cognetivity’s ICA, obtained in a multinational cohort of over 200 participants, demonstrated ICA’s suitability for rapid, population-wide deployment across the globe, including risk-based screening in primary care. Ultimately, Cognetivity’s ICA is not confined by international barriers like language, and has the potential to reach a limitless global market.
Furthermore, combined with artificial intelligence (AI), Cognetivity’s ICA has the potential to identify individuals in need of treatment as well as those most likely to benefit from a particular type of pharmacological intervention, regardless of their geographic origin. With this in mind, the ICA is also capable of measuring cognitive performance remotely without loss of testing accuracy, showcasing the platform’s functionality as a high-frequency monitoring tool both in the clinic and in a patients home, if necessary. As a result, ICA is well positioned to meet the increasing need for high-quality remote cognitive assessment spurred on by the COVID-19 pandemic.
“The diagnostic accuracy of the ICA and its novel use of explainable AI, combined with the power to generalize across other languages and cultures, make it uniquely suitable for cognitive screening across large and diverse populations. And in light of the FDA’s recent approval of the disease-modifying drug aducanumab, the need for a device capable of screening a wide population of at-risk individuals has never been higher,” commented renowned geriatric psychiatrist Professor Dag Aarsland, who co-authored the paper.
Aduhelm (aducanumab)
In addition to Cognetivity’s latest announcement, the FDA has approved Aduhelm (aducanumab) to treat patients with Alzheimer’s Disease using the Accelerated Approval pathway, which enables the FDA to approve a drug for a serious or life-threatening illness that may provide meaningful therapeutic benefit over existing treatments. With this in mind, Aduhelm has demonstrated its effectiveness in substantially reducing the level of amyloid beta plaques in the brain. For context, the beta-amyloid protein is a naturally occurring protein. However, in a brain affected by Alzheimer’s these proteins clump together forming a plaque between neurons, ultimately disrupting cell function.
Prime Time for Cognetivity
With a new Alzheimer’s treatment drug on the block, Cognetivity’s ICA could become a hot sell as the demand for capable screening tools increases. Moreover, Cognetivity is positioned nicely to capitalize on this opportunity according to the Company’s Financial Results for the years ended January 31, 2021 and 2020. In January 2021, Cognetivity was able to increase its total assets to CAD$1,827,799 up from $748,065 the previous year. The Company was also able to reduce its liabilities to $964,888 from $1,011,100 in the same period. Furthermore, by the end of January 2021, Cognetivity was able to more than double its cash from $578,299 to $1,359,851.
If you ask me, I think Cognetivity could see additional growth following the FDA’s approval of Aduhelm. The Company’s ICA now has an academic publication under its belt, further supporting that platforms credibility and exposure. Paired with the ICA’s early detection capabilities, Aduhelm may be able to produce significant results in treating Alzheimer’s Disease. As someone with a history of Alzheimer’s in my family, I sincerely hope that a tool like ICA reaches the spotlight in my lifetime as the prevalence rate of the disease continues to increase yearly.
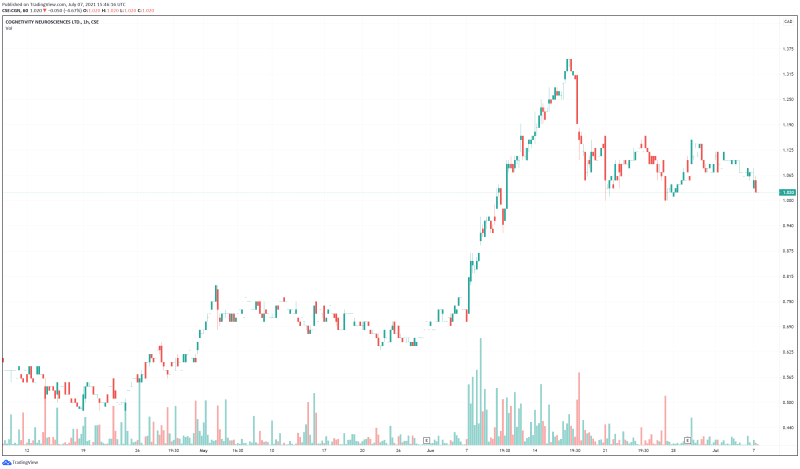
Cognetivity’s share price opened at $1.03, down from a previous close of $1.07. The Company’s shares are down 4.67% and are currently trading at $1.02 as of 11:41AM ET.