Bioasis Technologies (BTI.V) announced positive results today from an efficacy study of a blood-brain barrier penetrant interleukin-1 receptor inhibitor in a preclinical rodent model of multiple sclerosis (MS).
“These data further confirm the ability of the xB3™ delivery platform to enhance the delivery of large molecule therapeutics into CNS compartments with demonstrated disease-relevant efficacy. We are progressing these data and believe that xB3™-IL-1RA holds promise for the treatment of a broad range of neuroinflammatory diseases,” said Dr. Deborah Rathjen, Executive Chair of the Board of Bioasis.
Bioasis proprietary platform technology is intended to enhance the delivery of biologics across the blood-brain barrier (BBB) for the treatment of central nervous system (CNS) disorders in areas of high unmet medical need, including brain cancers and neurodegenerative diseases. For context, the blood-brain barrier is a barrier between the brain’s blood vessels and the cells and other components that make up brain tissue. The BBB plays a pivotal in protecting the brain from toxins and pathogens while permitting access to crucial molecules like oxygen and glucose.
With this in mind, in order to treat diseases of the central nervous system, treatment needs to be able to pass through the BBB’s strict defenses. Unfortunately, more than 98% of small molecule drugs and almost 100% of larger biologic drugs are unable to enter the brain at sufficient concentrations to cause therapeutic effects. Quite the conundrum, however, Bioasis’ patented xB3 platform has shown high efficacy in its ability to shuttle molecules across the blood-brain barrier, reaching the necessary targets within the brain. An arduous journey to pass through a seemingly impenetrable wall…sounds like a potential Osmosis Jones sequel in the making.
Movie references aside, in a previously published work, a recombinant fusion protein of the Company’s xB3 peptide with an interleukin-1 receptor antagonist (xB3 -IL-1RA) demonstrated efficient delivery of effective concentrations of IL-1RA to the brain, ultimately eliciting analgesia in a neuropathic pain animal model. Translated into English, xB3 -IL-1RA was able to successfully move high enough concentrations of IL-1RA through the BBB to elicit pain reduction.
Regarding Bioasis’ latest study, the Company’s xB3 -IL-1RA was investigated in a rodent experimental allergic encephalomyelitis (EAE) model, the most common experimental model for MS. During this study, xB3 -IL-1RA was https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered during the disease induction phase, resulting in both a delayed onset and overall reduced clinical symptom score in animals treated with xB3 -IL-1RA compared to control animals. Put simply, the Company’s latest results have provided further evidence supporting xB3 -IL-1RA’s ability to deliver large molecule biologics across the BBB. In particular, this study confirms the potential of xB3 -IL-1RA for treatment of neuroinflammatory diseases such as MS.
Overall, Bioasis is sitting pretty, having more than doubled its cash and cash equivalents year-over-year from $576,371 to $2,736,735 in February 2021. As evidence continues to mount in support of xB3 -IL-1RA, Bioasis’ patented technology platform could provide a key into the BBB, providing access to treatments that were previously locked out.
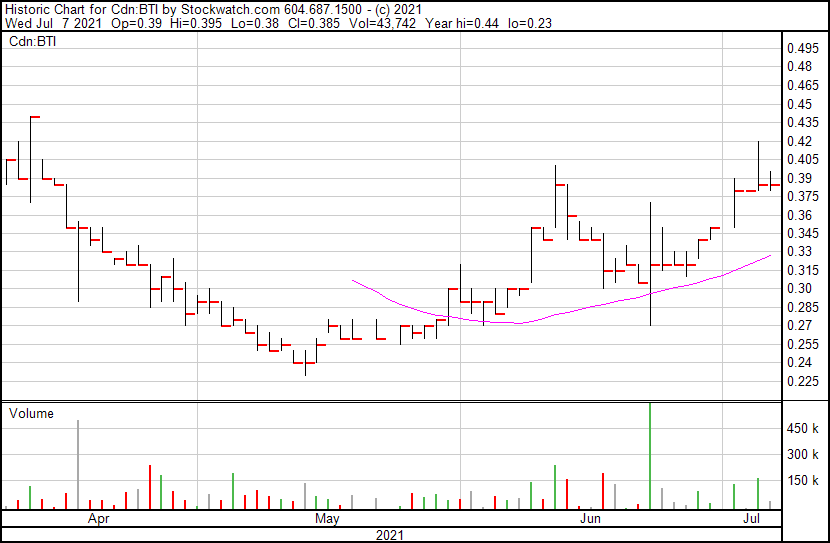
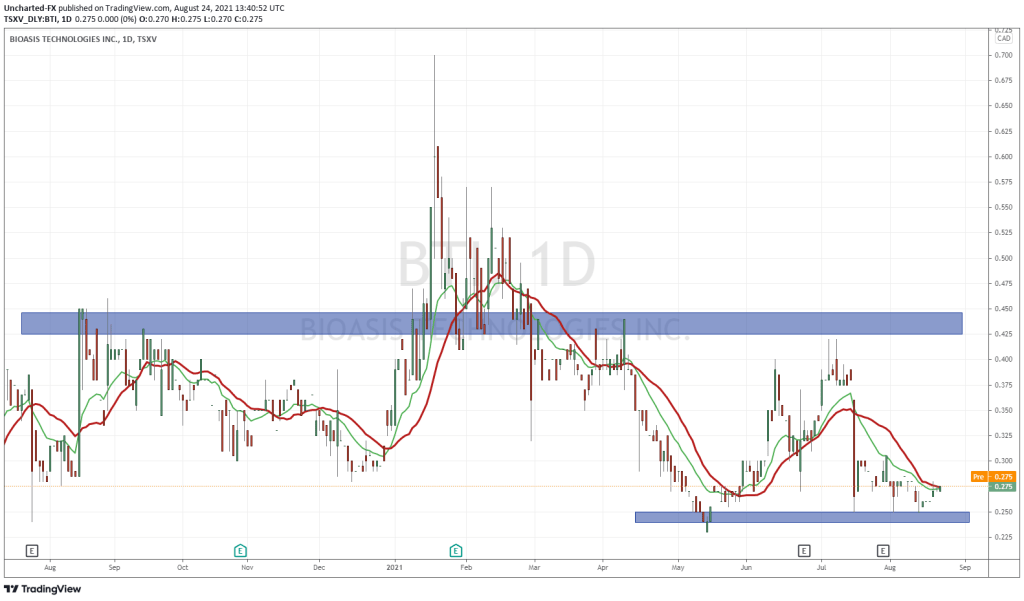
Bioasis share price opened at $0.39, up from a previous close of $0.385. The Company’s shares are currently trading at $0.385 as of $3.40PM ET.
Full Disclosure: Bioasis is marketing client of Equity Guru.