Histogen (HSTO.Q), and its partner Amerimmune, today announced top-line results from its Phase 1 study of emricasan in mild symptomatic COVID-19 patients to assess safety, tolerability, and preliminary efficacy.
“These positive results further reinforce the extensive clinical safety database of emricasan and strongly suggest that emricasan can potentially be developed as a therapeutic treatment for mild to moderate COVID-19, as well as other viral inflammatory diseases…We look forward to working with our colleagues at Amerimmune as we chart a strategic course for emricasan and its clinical development options and partnering opportunities,” said Richard W. Pascoe, Histogen’s President and Chief Executive Officer.
Time for a brief history lesson. Emricasan was invented in 1988 by Idun Pharmaceuticals and was acquire by Pfizer in 2005. Emricasan was then sold to Conatus Pharmaceuticals in 2010, however, Histogen completed a merger with Conatus on May 27, 2021. As a result, Histogen acquired certain rights to emricasan. For context, emricasan is a potential first-in-class, orally active, pan-caspase inhibitor designed to reduce the activity of enzymes that mediate inflammation and apoptosis, which refers to cell death. Put into layman’s terms, caspases and apoptosis are generally bad and emricasan is able to combat these issues. With this in mind, COVID-19 has been shown to activate caspases to trigger cell apoptosis in the lungs.
Histogen commenced its Phase 1 study of emricasan earlier this year on after dosing its first patient on March 16, 2021. In total, this double blinded, 1:1 randomized, placebo controlled single site study is expected to enroll 40 symptomatic mild-COVID-19 patients in an outpatient setting. Wow that’s a mouthful. Put simply, during Histogen’s clinical trial, both patients and researchers will be unable to determine which treatment participants are receiving until after the trial has concluded. Furthermore, Histogen’s Phase 1 study will be conducted as a single-site clinical trial, meaning one investigational site will be used to perform the study. With this in mind, SUNY Downstate Medical Center in Brooklyn, New York, has been selected as the single site for this study.
Histogen’s Phase 1 study uses emricasan at 25mg, taken twice a day, for 14 days versus placebo. In addition to testing safety and tolerability, this study also includes various clinical and laboratory measures and patient reported outcomes (PROs) using the *inhales* FDA COVID-19 Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment Assessment tool.
“The study data not only showed complete and early resolution of COVID-19 related symptoms in the emricasan group compared to the placebo group, which continued to have symptoms, but also provided substantial insight into disease mechanism, which will be critical in developing therapeutic options for COVID-19. We are excited to work with Histogen to bring Amerimmune’s expertise to the emricasan development program as a demonstration of how physician-owned diagnostic laboratories can be the genesis of breakthroughs in medicine and participate fully in the advancement of novel therapies,” said Dr. Oral Alpan, M.D. Chief Executive Officer of Amerimmune LLC.
According to Histogen’s latest announcement, this study has demonstrated that emricasan was safe and well tolerated during the 14 days of dosing and at the day 45 follow-up, as compared to placebo with no reports of serious adverse events. More notably, patients who completed treatment with emricasan experienced a complete resolution of symptoms most commonly associated with COVID-19, including coughing, headaches and fatigue. Patients were relieved of these symptoms after 7 days, none of which resurfaced during the 45 day period. On the other hand, patients who received placebo did not experience COVID-19 symptom resolution at any point during the same 45 day period.
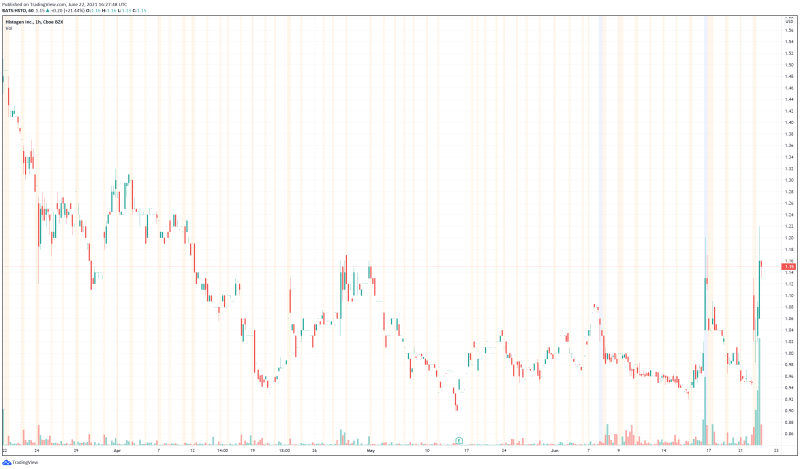
Histogen’s share price opened at $1.01, up from a previous close of $0.94. The Company’s shares are up 19.32% and are currently trading at $1.12. This indicates that there has been significant change following the news.

