Entera Bio (ENTX.Q) has announced the final 6-month bone mineral density (BMD) results from its Phase 2 clinical trial of EB613 for the treatment of osteoporosis.
“We are very excited and encouraged by these great results which will support advancing discussions with potential strategic partners. These results are in line with our previously reported biomarker results and further validate Entera’s platform technology and its potential to enable oral formulation of various large molecules for a range of indications that could benefit from an oral drug…, commented Spiros Jamas, CEO of Entera Bio.
Osteoporosis is a disease characterized by low bone mass and deterioration of bone tissue, which can lead to increased risk of fracture. To make matters worse, bone deterioration can occur over a number of years without presenting any symptoms. Furthermore, osteoporosis is responsible for approximately 2 million fractures annually, and is expected to incur yearly treatment costs of $25.3 billion by 2025. According to the National Osteoporosis Foundation (NOF), an estimated eight million women already have osteoporosis, and another 44 million may have low bone mass placing them at increased risk for osteoporosis.
With this in mind, Entera is a leader in the development of orally delivered large molecule therapeutics for use in areas with significant unmet medical needs. EB613 represents one of the Company’s most advanced product candidates and is intended for the treatment of osteoporosis. Using Entera’s proprietary, oral drug delivery technology, EB613 is is https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered orally and is positioned as the first potential once daily, bone building treatment for osteoporosis patients.
“We are looking forward to an end of Phase 2 meeting with the FDA. More detailed results will also be presented in a future scientific conference and publications. The company will evaluate potential additional osteoporosis market opportunities specifically related to increases in hip BMD,” continued Mr. Jamas.
Entera’s Phase 2 clinical trial of EB613 was a 6-month double blinded blind, dose-ranging, placebo-controlled study in 161 postmenopausal female subjects with osteoporosis, or low BMD. This study was intended to evaluate the efficacy and safety of varying doses of EB613. With this in mind, subjects who received the 2.5 mg dose of EB613 for 6 months had a significant placebo adjusted increase of 3.78% in lumbar spine BMD. Furthermore, the study’s primary efficacy endpoint, a statistically significant increase in P1NP at 3 months, was achieved. Overall, EB613 has exhibited an excellent safety profile, with no drug related serious adverse events.
Teriparatide for injection, marketed under the brand name Forteo®, has become the standard for osteoporosis treatment, however, Entera’s EB613 has demonstrated superior effectiveness in some areas. For example, in the Company’s Phase 2 clinical trial, EB613 was observed to have had a significant impact on both femoral neck and total hip BMD at 6 months. In contrast, significant increases in BMD of the femoral neck and total hip are usually not seen with Forteo treatment at 6 months.
It is worth noting that the global osteoporosis drugs market was valued at USD$13.36 billion in 2020. Moreover, this market is projected to grow at a CAGR of 6.83%, growing to approximately $20.17 billion by 2026. With this in mind, Entera shows potential as a treatment alternative to Forteo. If Entera can establish its EB613 as a staple in the osteoporosis drugs market, the Company could very well see its shares skyrocket. With this in mind, Entera shows potential as a profitable biopharmaceutical investment.
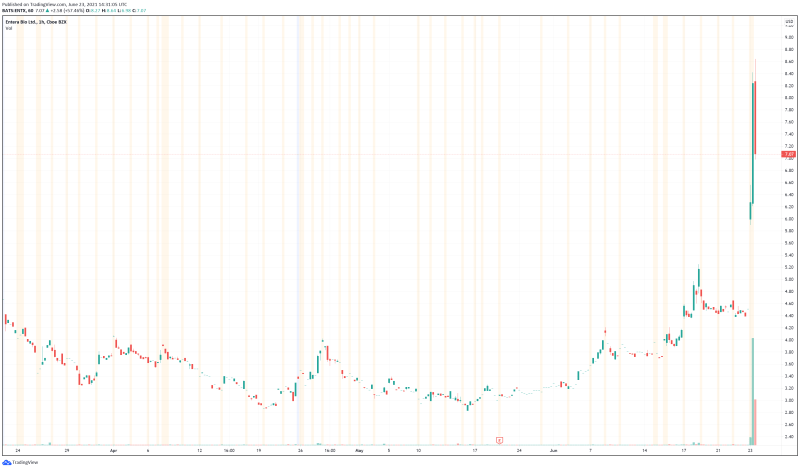
Entera’s share price opened at $7.21, up from a previous close of $4.49. The Company’s shares are up 65.25% and are currently trading at $7.36 as of 10:43AM ET. This indicates that there has been significant change following the news.