Mountain Valley MD Holdings (MVMD.C) filed a cancer patent and is moving forward with preclinical trials with third party cancer research organizations (CROs) in triple-negative breast cancer, metastatic melanoma and lung carcinoma, according to a press release.
The patent is called Novel Injectable, Infusable, Instillable Ivermectin Adjuvant for Cancer Therapies, and involves solubilized ivermectin (also known as Ivectosol). The secret to what this therapy does is in the word ‘adjuvant.’ An adjuvant is a substance that enhances the immune system’s response to the presence of an antigen. They’re commonly used to help vaccines do their work, and are injected alongside an antigen to help the immune system develop the antibodies to fight the disease.
“Imagine what is possible when you have the world’s only human injectable form of ivermectin that can be directly injected into a tumour or provided through more bio-available forms such as intravenously. We believe this will be groundbreaking research with near-immediate application to be able to proceed directly to human trials based on the safety and efficacy of ivermectin,” said Mike Farber, director of life sciences at Mountain Valley MD.
Mountain Valley MD is a biotechnology and life sciences company focused on the implementation of their patented Quicksome oral drug formulation and delivery system, as well as its Quicksol solubilization tech for macrocyclic lactones, the family of drug where ivermectin comes from.
If you’ve heard of ivermectin before but can’t pinpoint what it is or where you’ve heard it, then you probably have kids, or maybe some interesting stories yourself. It’s a medication used primarily to treat parasitic infestations. In humans that means head lice, scabies and other such maladies. In veterinary medicine, it’s for heartworm. It’s either taken orally or applied as a topical for external infestations.
This version of it is injectable, and according to a citation in the January 21 edition of Pharmacological Research, possesses powerful anti-tumour effects in a variety of cancer cells, as well as promotes the death of cancer cells, including apoptosis (programmed cell death), autophagy (or body’s natural processes of cleaning out old cells) and necrosis (through injury, usually). The research also noted how ivermectine had been shown to slow tumour stem cells and reverse multidrug resistance.The preclinical trials are designed to prove that Invectosol works with and improves upon existing cancer regimens, and also as a potential bolstering agent to immunotherapies and chemotherapies for hard to treat cancers.
Here are the specifics of the preclinical trials:
Study 1: triple-negative breast cancer
- Study will test the effectiveness of Ivectosol combined with checkpoint inhibitor that would be equivalent to either OPDIVO or Keytruda for disease progression and complete response rate;
- Arms in the study will look at single checkpoint inhibitor, oral IVM plus checkpoint inhibitor and intratumoural IVM plus checkpoint inhibitor;
- Estimated initial readouts/analysis — second week of June, 2021;
- Complete readout with flow cytometry and statistical evaluation estimated mid-July, 2021, with possible abstract submission in August, 2021.
Study 2: metastatic melanoma
- Study will test the effectiveness of Ivectosol intratumoural combined with checkpoint inhibitor for disease progression and complete response rate;
- Arms in the study will look at single checkpoint inhibitor, oral IVM plus checkpoint inhibitor and intratumoural IVM plus checkpoint inhibitor;
- Estimated initial readouts by end of June, 2021;
- Complete readout with flow cytometry and statistical evaluations estimated end of July, 2021, with possible abstract submission in August, 2021.
Study 3: Lewis lung carcinoma as a proxy for non-small cell lung carcinoma
- Study will test the effectiveness of Ivectosol intratumourally combined with checkpoint inhibitor for disease progression and complete response rate;
- Arms in the study will look at single checkpoint inhibitor, oral IVM plus checkpoint inhibitor, intratumoural IVM plus checkpoint inhibitor and navalbine plus intratumoural IVM;
- Estimated initial readouts by end of June, 2021;
- Complete readouts with flow cytometry and statistical evaluation estimated by end of July, 2021, with possible abstract submission in August, 2021.
All three preclinical studies will focus on tumour growth and metastases through bioluminescence imaging, which is a non-invasive optical imaging technique invented to observe and measure bioluminescent signals in tissues.
“Ivermectin is a Nobel-prize-winning global blockbuster drug with unprecedented potential. Overcoming its No. 1 limitation of solubility using FDA-approved excipients has opened up significant applications across multiple human and animal health lanes for Mountain Valley MD and our partners. Driving innovation in cancer research to support positive outcomes for increased survival rates, productivity and improved quality of life for the global population is directly aligned with our mission of more life, less death. We couldn’t be more honoured to drive forward this important work,” said Dennis Hancock, president and chief executive officer of Mountain Valley MD.
Cancer is the leading cause of death worldwide, according to the World Health Organization. It killed nearly ten million people in 2020 alone. The World Cancer Day foundation estimates the annual economic cost to be around USD$1.16 trillion.
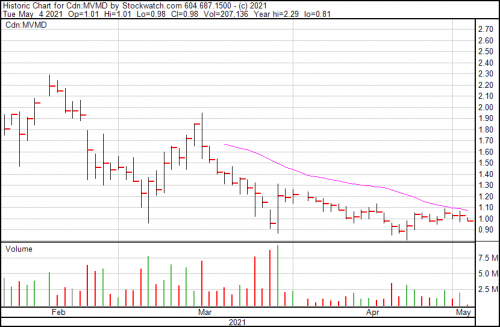
Mountain Valley is down $0.05 today and trading presently at $0.98.
—Joseph Morton
Full disclosure: Mountain Valley MD Holdings is an equity guru marketing client.