DMT biz dev
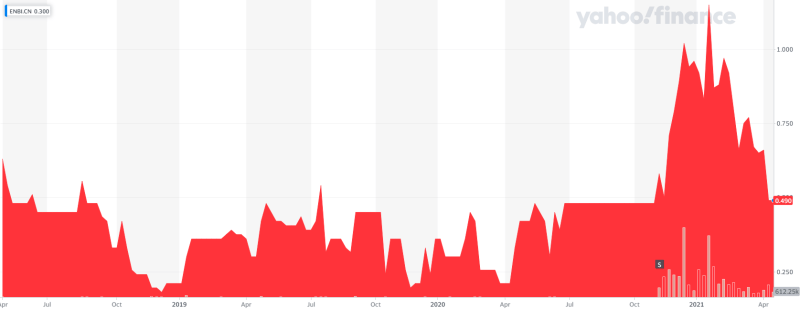
Entheon Biomedical (ENBI.C) is going all-in on the DMT (dimethyltryptamine) game. They are the only DMT-focused company targeting addiction.
The company is pre-revenue with phase 1 trials coming down the pike this year. The company recently developed a genetics test kit for psychedelic trips which I wrote about previously. But the main focus of Entheon is on DMT products and DMT-assisted therapies for addictions.
Entheon believes there is enough evidence out there to support targeting substance use disorder and smoking addiction through DMT-assisted treatment. Johns Hopkins and MAPS have laid the groundwork in their previous studies examining psilocybin and ayahuasca for smoking addiction and substance addiction.
Ayahuasca is a psychotropic brew originating from South America. The Amazonian vine Banisteriopsis caapi and leaves of the bush Psychotria viridis contain harmala alkaloids and DMT. When both are taken in combination it induces several hours of dream-like states producing auditory, ideational, and emotional effects typically lasting around 8 hours. The effects of DMT on its own last only for a small fraction of that time.
Entheon entered into a supply agreement with Alberta-based psychedelics company Psygen. The agreement outlines that Psygen will provide Entheon with GMP and non-GMP quality DMT drug products and substances for its preclinical, clinical trials. Their initial order was for $40k USD.
Entheon is not, and does not intend, to produce the DMT that it will utilize in the DMT products and in the preclinical and clinical studies leading up to commercialization, which will be up to Psygen.
Entheon entered into a supply agreement with CHDR (the Centre for Human Drug Research located in Leiden, Netherlands) to perform a DMT-based phase I safety and proof-of-concept clinical study in humans. Entheon will pay $1.4M to CHDR for the phase I study. In the future, Entheon intends on developing DMT Products in a form that can be https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered by intravenous therapy or other routes in clinical trials. The company has filed 4 patents for its DMT delivery systems.
https://equity.guru/2021/03/11/why-companies-like-mindset-pharma-mset-ne-are-adamant-on-synthesizing-psilocybin-mushrooms/
Further development of the DMT Products to a commercialization stage will be ongoing and dependent on the dosing strategies data obtained in planned phase I and 2 clinical trials.
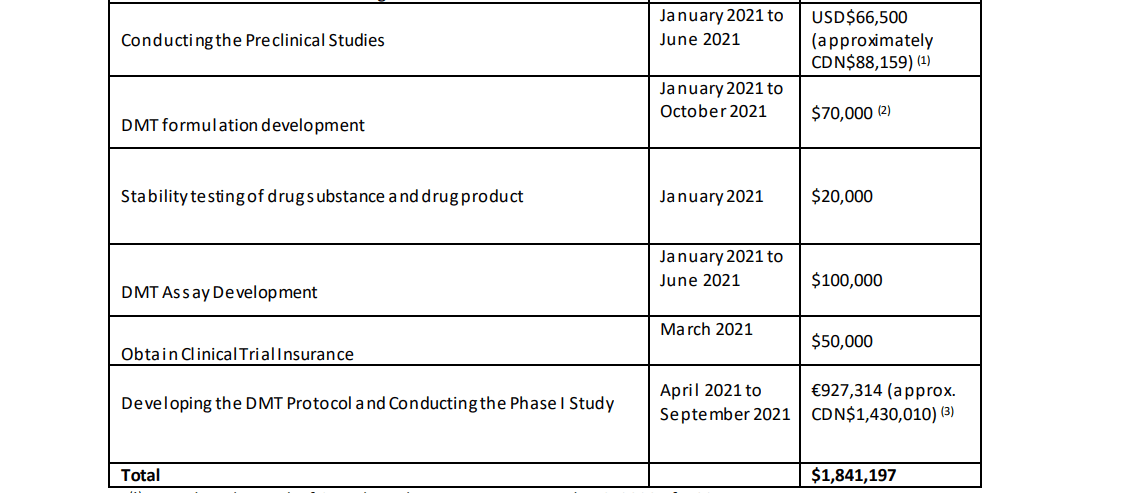
Entheon currently has approximately $4.2M CAD in working capital. The cost projections for its DMT products and studies are as follows:
Upon completion of, among other things, the Phase I Study, Entheon intends to complete an additional clinical study in the United States focused on addressing nicotine addiction
The Phase 2 Nicotine Study is expected to compare measures of neuronal activity and complexity between subject groups, obtain cardiovascular and immunological health data, assess improvements in objective measures of wellbeing via interviews and questionnaires, evaluate frequency and severity of adverse effects, and determine the rate of nicotine use following DMT-assisted therapy.
The Phase 3 Study consists of an expanded pilot study to multiple clinics to assess treatment outcomes in larger populations of nicotine users, including specific indications.
Johns Hopkins & MAPS
In 2014, Entheon advisor Dr. Matthew Johnson and his colleagues at Johns Hopkins University published the results of their study measuring the safety and efficacy of psilocybin in treating tobacco addiction/ The study was inspired in part by research on psychedelics from the 1950s-70s that showed hope in treating alcohol and opioid dependence. The participants of the Hopkins study received 15 weeks of CBT therapy in combination with 2-3 psilocybin sessions.
At the 6-month follow-up, 80% of the participants were abstinent based on both self-reports and the presence of cotinine in urine and exhaled carbon monoxide, both are byproducts of nicotine and evidence of a recent smoking relapse. A longer-term follow-up study showed 67% of the participants remained abstinent after 12 months.
Dr. Johnson’s team at Johns Hopkins is currently conducting a study o 80 treatment-resistant, nicotine-dependent subjects. The trial is comparing a single high dose psilocybin session to an 8 to 10-week standard course of nicotine patches, both will incorporate 13 weeks of CBT therapy.
In 2013, MAPS completed the first-of-its-kind study using ayahuasca to investigate treating substance abuse disorders. The subjects included 12 members of a rural First Nations community, several of whom had been through numerous unsuccessful treatments.
The treatment consisted of a four-day retreat facilitated by Dr. Gabor Mate. The study combined four days of group counseling with two expert-led ayahuasca ceremonies. Group counseling sessions consisted of various psychosomatic techniques mixed with group sharing and dialogue.
The study’s findings showed statistically significant improvements that were demonstrated through assessing hopefulness, empowerment, mindfulness, and quality of life meaning and outlook subscales.
https://equity.guru/2021/02/24/new-innovations-in-schizophrenia-related-treatments-via-psychedelics/
Self-reported alcohol, tobacco, and cocaine use declined, while cannabis and opiate use did not. But the reported reductions in problematic cocaine use was the most statistically significant outcome of the study. According to MAPS, these findings suggest participants may have experienced positive psychological and behavioral changes in response to this therapeutic approach, and that more rigorous research of ayahuasca-assisted therapy for problematic substance use is warranted.
Worldwide?
With two of the most trusted names in psychedelics – MAPS and John’s Hopkins getting the ball rolling on DMT therapy for addictions, Entheon is a good spot. They are a unique company in a fast-growing sector. Based on previous studies their business plan has hope. This isn’t a sure thing like a MindMed (MMED.NEO) Compass Pathways (CMPS.Q), Entheon still has a long way to go in proving itself. And unlike psychedelics companies Field Trip (FTRP.C) and Novamind (NM.C), Entheon is pre-revenue.
https://equity.guru/2021/03/01/field-trip-ftrp-cn-is-scaling-its-posh-ketamine-therapy-business-after-huge-ass-95m-raise/
Along with their own clinical trials, more studies like the one currently being done at Johns Hopkins could help solidify Entheon’s vision even further. Their goal is to ultimately get market authorization from Health Canada, the FDA, and the EMA to sell its DMT Products in Canada, the United States, and the European Union. However, prior to doing so, Entheon will need to go through the clinical study regulatory process to have its dosing strategies approved.
However, with their test kits and supply agreements, there could be revenue coming in soon. Whether or not it’s significant revenue, Entheon has big plans for the future.