On February 24, 2021, XPhyto Therapeutics (XPHY.C) announced that it has placed the first order for its rapid point-of-care SARS-CoV-2 (COVID-19) RT-PCR test system (Covid-ID Lab) with its exclusive diagnostic development partner, 3a-diagnostics GmbH.
“PCR tests are used to directly detect the presence of an antigen, rather than the presence of the body’s immune response, or antibodies,” states Medical Device Network, “By detecting viral RNA, which will be present in the body before symptoms of the disease are present,” the tests give a powerful early warning system.
Xphyto – a bioscience accelerator – has already completed validation of the Covid-19 PCR Diagnostic Test Kit.
The PCR test system demonstrated clinical accuracy in detecting SARS-CoV-2 RNA within 25 minutes, making it an ideal screening tool for international airports.
The first order of Covid-ID Lab is for 9,600 individual tests, which are packaged in 200 kits of 48 tests each.
XPhyto and 3a-diagnostics GmbH have signed an “exclusive development, technology purchase, and licence agreement” for the development and commercialization of rapid, point-of-care, low-cost, and easy-to-use infectious disease screening tests.
Unlike Vektor Pharma and Bunker – 3a-diagnostics GmbH is XPHY’s “commercial partner” – not a 100%-owned subsidiary.
Delivery of the first order is expected by mid-March 2021.

What does this non-arms length transaction achieve?
“The 1st order will be primarily used to supply prospective distribution partners and licensees and their respective government regulators with test samples for review and evaluation,” explains Xphyto.
The tests will be manufactured in the German state of Baden-Württemberg.
“We are pleased to report that all steps towards the launch of Covid-ID Lab remain on track within an ambitious timeline,” stated Hugh Rogers, CEO, and Director of XPhyto.
Linking a new medical product with purchasers is not something you accomplish by Googling “Biggest Buyers of Covid-19 PCR screen tests”.
You need a vet executive team with high-level relationships in the pharmaceutical community.
“Our experienced market launch team is working quickly to bring the product to market, as well as to establish licensing and distribution partnerships,” stated Rogers.
Three days ago we reported that XPhyto Therapeutics (XPHY.C) was fast-tracking the assembly of an elite commercial team to launch its point-of-care COVID-19 RT-PCR test system.
“The market launch team consists of world-class clinical and pharmaceutical executives and service providers who bring the experience and expertise necessary to effectively and rapidly drive the commercialization of COVID-ID Lab”.
Collectively, Xphyto’s commercial team brings decades of experience in the biotech and pharmaceutical industries, including:
- Executive leadership
- Business development
- Regulatory compliance
- Product launch
- International marketing
- International sales
- Production
- Quality assurance
- M&A (mergers and acquisitions).
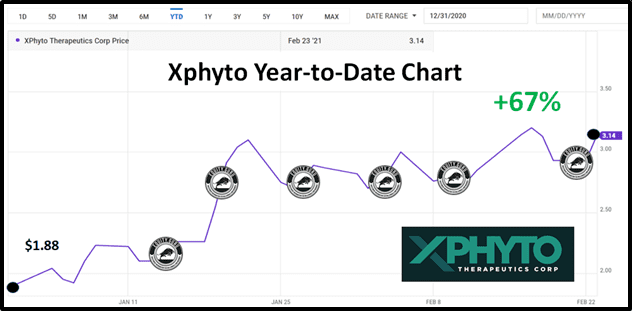
In 2021, Xphyto stock price has risen from $1.88 to $3.14 as Equity Guru has covered a barrage of XPHY’s stated and achieved milestones.
- Jan 18, 2021: Xphyto Therapeutics (XPHY.C) lays out their milestones and strategic guard-rails for 2021
- Jan 22, 2021: VIDEO: First Glance with Jody Vance Ep14 – We talk to Xphyto Therapeutics (XPHY.C) CEO and director Hugh Rogers
- Jan 28, 2021 Xphyto’s (XPHY.C) lays out its ambitious Parkinson’s/Epilepsy/Depression/Multiple Sclerosis drug formulation strategy which includes heavy investment in psychedelic medicine
- Feb 2, 2021: Xphyto Therapeutics (XPHY.C) deal with Applied Pharmaceutical Innovation prepares for future supply woes
- Feb 10, 2021: Xphyto Therapeutics (XPHY.C) adds mescaline to Applied Pharmaceutical Innovations psychedelic target list
- Feb 22, 2021: Xphyto (XPHY.C) assembles biotech/biz dream-team to launch 25-minute Covid-19 test
“The process of team building” states Talking Talent, “involves establishing clear objectives, demonstrating strong leadership, incentivizing team-members, creating a ‘big picture’, delegating tasks, giving feedback and scheduling team-building exercises.”
Apparently Xphyto didn’t get the message.
When – according to “Talking Talent” – Xphyto’s embryonic commercial team should be sharing virtual cupcakes and white-boarding inspirational slogans – it will be delivering PCR test kits to prospective distribution partners and licensees.
Covid-ID Lab was designed to be a rapid, accurate, and robust COVID-19 test system with reduced operating costs and increased convenience and portability.
Roll-out Timeline:
- End of February, 2021: XPhyto expects 3a to receive ISO 13485 medical device manufacturer approval.
- Early March: European regulatory approval as a commercial in vitro diagnostic device (CE-IVD) for Covid-ID Lab
- Initial commercial manufacturing is planned for Germany, with additional capacity in other jurisdictions to follow.
- April, 2021: The sales launch in Europe.
XPhyto is currently in discussions with potential distribution and wholesale partners in Europe and the Middle East.
Xphyto and 3a are also developing a portfolio of oral biosensor screening tests for detection of bacterial and viral infectious diseases, including influenza A, group A strep, stomatitis, periimplantitis and periodontitis.
Additional pandemic-focused biosensors are in development, specifically for H1N1 (swine flu) and H5N1 (avian flu). The company is planning commercial launch of its first biosensor product in the second half of 2021.
Equity Guru’s Jody Vance recently spoke with Xphyto’s CEO and Director Hugh Rogers to get a bird’s eye view of the company, its portfolio and what the markets can look forward to over the next 12 months.
“The team has extensive experience from regulatory approval to international production and sales,” stated Rogers. “As we near expected regulatory approval, Xphyto’s business is rapidly evolving from innovation to impact.”
As a 2nd wave of Covid-19 sweeps across the globe, commercial product launch in Europe is planned for Q1 2021.
“We are confident that Covid-ID Lab, as a 25-minute PCR test with minimal technical and personnel requirements, will be a stand-out product in the COVID-19 test market,” added Rogers.
- Lukas Kane.
Full Disclosure: Xphyto is an Equity Guru marketing client







