Revive Therapeutics (RVV.C) advanced the December 21 deal with Newscope Capital (PHRM.C) past a binding LOI and into a purchase agreement to acquire psilocybin intellectual property from Newscope’s subsidiary, PharmaTher, today, according to a press release.
We originally covered this story when it dropped in December noting that the assets will include all the intellectual and work property, with the ultimate goal being Orphan Drug Designation from the U.S. Food and Drug Administration (FDA).
“With this acquisition, we have solidified our foundation in having a leading psychedelics pharmaceutical platform with a focus on proprietary psilocybin-based therapeutics that includes the development of an oral thin film product in collaboration with the University of Wisconsin-Madison, a novel biosynthetic version of psilocybin based on a natural biosynthesis enzymatic platform developed by Dr. Gavin Williams, Professor and Researcher at North Carolina State University, a clinical study with the University of Wisconsin evaluating psilocybin in the treatment of methamphetamine use disorder, and PharmaTher’s psilocybin research initiatives and intellectual property in stroke, traumatic brain injury, cancer and drug combinations. We are now in a position to advance our psilocybin program for future clinical development in various unmet clinical needs in mental health, cancer and neurological disorders,” said Michael Frank, CEO of Revive.
Revive is a life sciences company working on finding therapies for infectious diseases and rare disorders, and it’s geared its drug development track towards taking advantage of regulatory incentives offered by the FDA, like Orpan Drug, Fast Track, Breakthrough therapy and Rare Pediatric Disease. At present, they’re looking into Bucillamine as a potential treatment for infectious diseases, among them being COVID-19.
The purchase price is $10 million, spread out over three different distinct criteria. The first is $3 million in cash on the closing date, and another $4 million in Revive’s shares, and another $3 million in either cash or shares when and if Revive hits certain milestones, like receiving orphan drug designation for psilocybin to treat strokes, traumatic brain injuries or cancer, or the second phase of a clinical trial and regulatory filing for FDA approval.
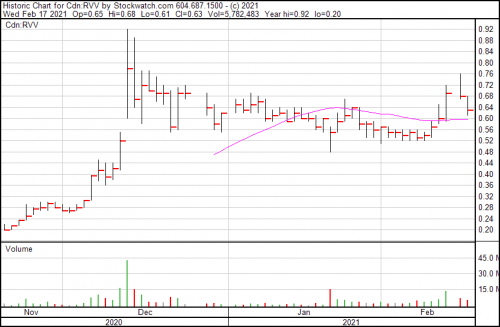
Shares dipped $0.05 today, and are presently trading at $0.63.
—Joseph Morton
Full disclosure: Revive Therapeutics is an equity.guru marketing client.