On September 29, 2020 Revive Therapeutics (RVV.C) updated the investment community on its U.S. Food & Drug Administration (FDA) phase 3 clinical trial to evaluate the safety and efficacy of bucillamine in patients with mild-moderate COVID-19.
RVV is a specialty life sciences company focused on the R&D of therapeutics for medical needs and rare disorders.
The company is benefiting from FDA regulatory incentives for Orphan Drugs, Breakthrough Therapies and Rare Pediatric Diseases.
With its recent acquisition of Psilocin Pharma Corp, RVV is advancing the development of Psilocybin-based therapeutics in various diseases and disorders.
Revive’s cannabinoid pharmaceutical portfolio received a boost when RVV was granted FDA orphan drug status designation for the use of Cannabidiol (CBD) to treat autoimmune hepatitis (liver disease) and to help heal injury from organ transplantation.
Bucillamine is a versatile organic molecule with an array of utilities including “regulating B-cell function” “thiol antioxidant” and “cisplatin-induced otoxicity”.
“Bucillamine has potential to attenuate or prevent damage during myocardial infarction, cardiac surgery and organ transplantation,” states the University of Colorado Health Sciences Center, it “enters the cells by the same mechanism that normally transports the amino acid cysteine”.
Preclinical and clinical studies have demonstrated that bucillamine can significantly reduce the negative symptoms of respiratory viral infections in animals and humans.
Revive has identified bucillamine as potentially useful in the treatment of COVID-19.
The FDA agrees with that assessment.
With the FDA’s approval, Revive has set-up five clinical sites in Florida, Texas and California for enrolment of patients in the phase 3 clinical study.
It is also finalizing agreements with an additional 10 clinical sites in these states, including Arizona and Ohio.
Patient enrolment is expected to start in the ten additional sites in October, 2020.
“We have made significant progress in advancing the phase 3 clinical trial since the FDA approval allowed us to proceed with the study,” stated Michael Frank, Revive’s CEO, “and we are expanding on and engaging with clinical sites in high-prevalence-COVID-19-infected states.”
Frank believes that RVV will meet its enrolment goals and expedite the potential FDA approval and commercialization of bucillamine for the treatment of COVID-19.

The “Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study of Bucillamine in Patients with Mild-Moderate COVID-19” is underway.
“Multi-center” means that the trial is carried out at more than one medical institution; “randomized” means that there will a control group https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered a placebo; “double-blind” means that neither the patients nor the scientists know who is receiving a particular treatment.
Phase 3 Clinical Trial Highlights:
- Enroll up to 1,000 patients
- Randomized 1:1:1 to receive bucillamine 100 milligrams three times a day, bucillamine 200 milligrams three times a day or placebo three times a day.
- 14-day trial
- Primary objective ito compare the frequency of hospitalization or death in patients with mild-moderate COVID-19 receiving bucillamine therapy with those receiving placebo.
- Trial will track hospitalization or death from the time of the first dose through Day 28 following randomization.
- Efficacy will be assessed by comparing clinical outcomes (death or hospitalization) amongst the three groups.
- Disease severity to be assessed using the 8-category NIAID COVID ordinal scale.
Safety will be assessed by comparing pre-treatment adverse events and treatment-emergent adverse events.
Revive has set up the trial so that it can be reactive to the data collected from the first wave of patients.
An interim analysis will be performed by an Independent Data and Safety Monitoring Board (DSMB) after 210 patients have been treated and followed up for 28 days after randomization.
Revive wants to find out quickly which dose of bucillamine is most effective.
“The better performing Bucillamine dose at the interim analysis will be selected and patients will then be randomized 2:1 to the selected Bucillamine dose or placebo,” states RVV.
In the video below (starting at the 30-second mark), Revive CEO Michael Frank discusses the evolution of Bucillamine in RVV’s development pipe-line.
Scientific Rationale of Bucillamine for COVID-19
Bucillamine has a well-known safety profile and is prescribed in the treatment of rheumatoid arthritis in Japan and South Korea for over 30 years.
The drug is non-toxic with high cellular permeability.
With 24.5 million confirmed cases of coronavirus globally, and 1 million dead, the total economic damage caused by the virus is projected to be between $8.1 and $15.8 trillion.
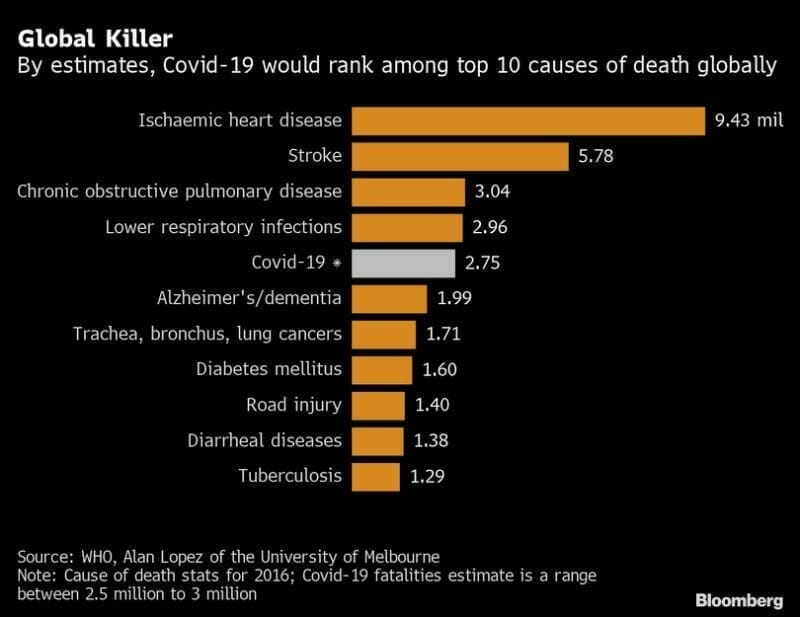
Given the grim human and financial data, it’s no surprise that biotech companies and their shareholders are focused on developing coronavirus solutions.
At this time, RVV is not making any express or implied claims that its product has the ability to eliminate or cure COVID-19 (SARS-2 Coronavirus).
- Lukas Kane
Full Disclosure: Revive Therapeutics is an Equity Guru marketing client.