Creative Medical Technology Holdings (CELZ.OTC) announced proof of concept studies to demonstrate the efficacy and use-value of their ImmCelz immunotherapy product for the treatment of rheumatoid arthritis today.
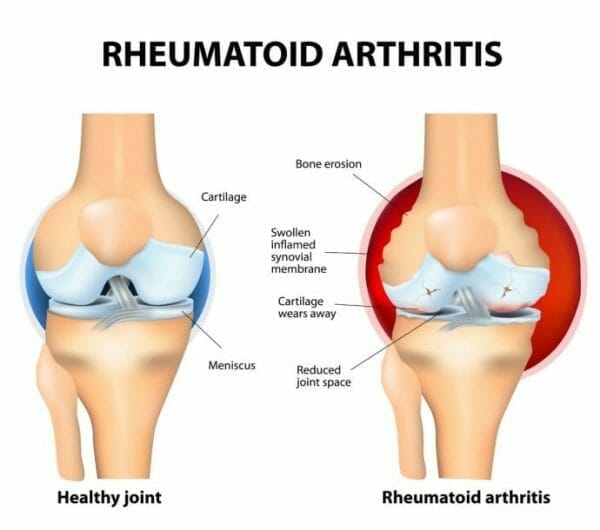
Rheumatoid arthritis happens when the body’s immune system views its otherwise healthy body tissue as being foreign invaders and sends out its usually immune response agents to attack. The result is pain and swelling in the wrist and small joints of the hands, feet and other joints. The disease is progressive, which means that acquiring it and not treating it properly generally leads to severe joints to the damage and serious complications to the major organs.
The studies involve exposing mouse immune cells to the company’s reprogramming protocol to show the selective suppression of autoimmunity, which causes damage to the joints while sparing normal immune functions. At present, treatments for RA aren’t specific in depressing immune responses, which leads to a perpetual condition of immunosuppression and increased susceptibility to infection.
“Previous publications have demonstrated ability to selectively suppress harmful immune responses using complicated gene modification technology. The data disclosed suggest ability of ImmCelz to achieve superior results in a manner which is amenable to rapid clinical translation,” said Timothy Warbington, CEO of Creative Medical Technology Holdings.
Creative Medical Technology Holdings has been around since 2011, focusing on regenerative medical solutions for otherwise unmet urological, neurological and orthopedic needs. They’ve developed a lengthy intellectual property portfolio using stem cells for the treatment of multiple medical issues, and have since patented, trademarked and commercialized products for everything from male and female sexual dysfunction, premature ovarian failure and pain management  degenerative disc disease. All of these use patient’s fresh and non-manipulated bone stem cells harvest from bone marrow, known in the business as autologous cells. They’re also working on expanding their intellectual property portfolio by using universal donor stem cells called allogenic stem cells. The product is called AmnioStem and deals specifically with stroke patients, using stem cells derived from amniotic fluid.
degenerative disc disease. All of these use patient’s fresh and non-manipulated bone stem cells harvest from bone marrow, known in the business as autologous cells. They’re also working on expanding their intellectual property portfolio by using universal donor stem cells called allogenic stem cells. The product is called AmnioStem and deals specifically with stroke patients, using stem cells derived from amniotic fluid.
ImmCelz is their latest, and the process goes like this:
ImmCelz uses adult stem cells from qualified donors to bestow specific properties on patient immune cells. After the immune cells have gone through an incubation period with the donor stem cells, the immune cells are re-injected back into the patient. These cells will then be considered “reprogrammed” and should then properly educate other immune cells to stop launching attacks on the body, while preserving the ability to attack cancers, foreign pathogens and anything else they should be attacking.
“Immunotherapy represents a revolution in medicine in which cells of the immune system are being developed as drugs. While majority of efforts are focused on stimulation of immunity, for treatment of cancer and viruses, our ImmCelz product represents a radically novel platform aimed at selective suppression of harmful immune response,” Warbington said.
Rheumatoid arthritis effects one out of every 100 Canadians, according to the arthritis society. A little quicky math and that factor expands out to include about 300,000 Canadians. Anyone can get it at any age, and RA affects women more than men to a factor of two to three times more. This and other harmful immune responses, collectively called autoimmunity, represent a multi-billion dollar market. Other conditions include type 1 diabetes, multiple sclerosis, lupus, RA and Grave’s Disease.
“It is our goal to continue to bring our robust Allogenic Stem Cell programs through patent issue, thus creating a barrier of entry to competition, compilation of data for proof of concept, early stage trial(s) via FDA Investigational New Drug applications (IND) and to continue to seek out collaborators, potential licensees and/or joint venture partners from larger life science companies within our space,” said Donald Dickerson, the company’s chief financial officer.
—Joseph Morton